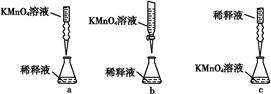
��Ŀ����
��������Ҫ�ɷ�ΪFeS2�����ҹ���������᳧��ȡ�������Ҫԭ�ϡ�ij��ѧѧϰС���ij������ʯ��������ʵ��̽����
[ʵ��һ]�ⶨ��Ԫ�صĺ�����
��m1 g�û�������Ʒ��������ͼ��ʾװ�ã��гֺͼ���װ��ʡ�ԣ���ʯӢ���У���a�����ϵػ���ͨ���������������ʯӢ���еĻ�������Ʒ����Ӧ��ȫ��ʯӢ���з�����Ӧ�Ļ�ѧ����ʽΪ��4FeS2+11O2 2Fe2O3+8SO2
2Fe2O3+8SO2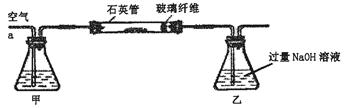
��Ӧ��������ƿ�е���Һ�������´�����
|

�������ۣ�
��1��I�У���ƿ����ʢ�Լ���____��Һ����ƿ�ڷ�����Ӧ�����ӷ���ʽΪ____��
��2��II�У���ƿ����H2O2��Һʱ��Ӧ�����ӷ���ʽΪ_____________________��
��3���û���������Ԫ�ص���������Ϊ_______________________��
��4��III�IJ�����У���Ҫ�õ����������ձ�������������ͷ�ι��⣬����____________________________________________��
��5��III�IJ�����У���ʾ�ζ��Ѵ��յ��������
��6����IJ���ܽ���������ƽ��ʵ�飬�������KMnO4��Һ����ֱ�Ϊ24.98mL��24.80mL��25.02mL��KMnO4����ԭΪMn2+���������������ݣ��ɼ�����û�������Ʒ��Ԫ�ص���������Ϊ ��
��1���������ƣ����������أ���2�֣� SO2+2OH��=SO32��+H2O����2SO32��+O2=2SO42��δд���۷֣���2�֣�
��2��SO32��+H2O2=SO42��+H2O ��2�֣�
��3��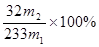 ��2�֣�
��2�֣�
��4��250ml����ƿ��2�֣�
��5�����һ�θ��������Һ����ʱ����Һ��ɫͻ��Ϊ��ɫ������30s�ڲ���ɫ����2�֣�
��6��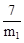 ��
��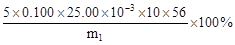 �����������𰸣�2�֣�
�����������𰸣�2�֣�
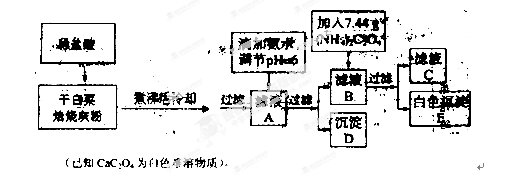
���������������1��Ϊ��ֹSO2���뵽��װ���У������������ƣ����������أ����գ���ƿ�е�������������Ӳ�ʲ������в����Ķ�����������Ӧ�����ӷ���ʽΪ��SO2+2OH��=SO32��+H2O��������ƿ��Ҳ������δ��ȫ��Ӧ����������Ҳ����2SO32��+O2=2SO42����2��II�У���ƿ����H2O2��Һʱ��Ӧ�����ӷ���ʽΪSO32��+H2O2=SO42��+H2O����3����ƿ�е���Һ����SO42����SO42���������Ȼ����е�Ba2+�������Ϸ���������Ӧ��SO42��+Ba2+=BaSO4����������m2gΪBaSO4����������й�ϵʽ���£�
FeS2��2SO42����2BaSO4
1mol 2mol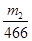
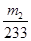
�ɴ˿�֪����Ԫ����FeS2�е����ʵ���Ϊ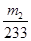 ����������Ϊ��
����������Ϊ��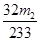 �����Ըû���������Ԫ�ص���������Ϊ
�����Ըû���������Ԫ�ص���������Ϊ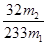 ��������4��ӦΪ250ml����ƿ����5����Ϊδ����KMnO4��Һ֮ǰ����Һ�к���Fe2+����ҺΪdz��ɫ��
��������4��ӦΪ250ml����ƿ����5����Ϊδ����KMnO4��Һ֮ǰ����Һ�к���Fe2+����ҺΪdz��ɫ��
���������һ�θ��������Һ����ʱ����Һ��ɫͻ��Ϊ��ɫ������30s�ڲ���ɫ����֤������ζ��յ㡣
��6��������Ӧ�Ļ�ѧ����ʽΪ5Fe2++8H++MnO4-=Mn2++5Fe3++4H2O,Ȼ�����5Fe2+��MnO4-�й�ϵʽ�����
�û�������Ʒ��Ԫ�ص���������Ϊ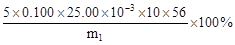 ��
��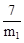
���㣺�������ʵķ������ᴿ����ѧʵ���������������ԭ��Ӧ�����ӷ���ʽ����д��������ԭ��Ӧ�ζ�ԭ��
����㡣

 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
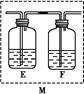
����һ������ܼƻ�ϵ�д�(14��)�������ҹ��ĺ�����ҵȡ���˾�ijɾͣ��ں��췢��ʱ����(N2H4)���������ﳣ��������ƽ�����
��Һ̬�������ȼ��ʱ����Һ̬N2O4��Ϸ���������ԭ��Ӧ����֪ÿ1 g�³�ַ�Ӧ��������̬ˮ�ų�����Ϊa KJ����д���÷�Ӧ���Ȼ�ѧ����ʽ ��
����ʵ�����У���N2H4��H2O��NaOH����һ�������ռ�114��116 �����ּ�Ϊ��ˮ�¡�
������������в���Ҫ�������� (�������ĸ)��
| A���ƾ��� | B����ֱ�������� | C����ƿ | D�������� |
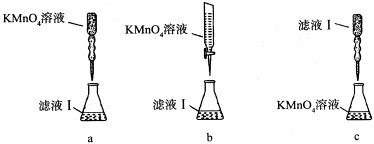
�ڳ���������������⣬��ȱ�ٵIJ��������� ��
������ʹ��¯�ڱڵ�����(��Ҫ�ɷ�Fe2O3)��ɴ���������(Fe3O4)�㣬�ɼ�����¯��ʴ������Ӧ��������ת��Ϊ��������ÿ����1 mol Fe3O4����Ҫ�����µ�����Ϊ g��
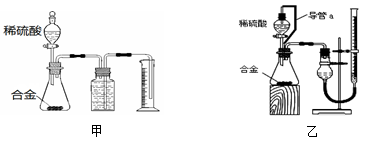
�ȴ���������(Fe3O4)����ɿ�д��FeO��Fe2O3��ij��ѧʵ��С��ͨ��ʵ����̽��һ��ɫ��ĩ�Ƿ���Fe3O4��CuO���(������������ɫ����)��̽���������£�
������裺����1. ��ɫ��ĩ��CuO������2. ��ɫ��ĩ��Fe3O4��
����3. ��
���̽��ʵ�飺
ȡ������ĩ��������ϡ�����У���������Һ�еμ�KSCN�Լ���
��������1��������ʵ�������� ��
����������Һ��Ѫ��ɫ������� ������
��Ϊ��һ��̽����������������Һ�����������ۣ������� �����������3������
����һС��ͬѧ��������������CuO�������٣����ܼ������ۺ�ʵ���������ԡ�
�������ϣ�Cu2����������ˮ��Ӧ��������ɫ��Һ��Cu2����4NH3��H2O��Cu(NH3)42����4H2O��
��Ϊ̽���Ǽ���2���Ǽ���3��������ȡ������ĩ��ϡ�������ܽ���ټ���������ˮ��������2����������� ���������� ���������3������
ijͬѧ��������ͭ����ᾧˮ�����IJⶨʵ�顣���������գ�
��ʵ�鲽�衿
��1����_______�����������ƣ���ͬ��ȷ������������������
��2���ڴ������м���Լ2 g��ϸ������ͭ���壬��������
��3����ʢ������ͭ����Ĵ������������������������ȣ�ֱ����ɫ��ȫ��ף�Ȼ�����������____________����ȴ�����£���������
��4���ظ�(3)��ʵ����к��ز�����ֱ�����γ������������0.001 g��
�����ݼ�¼�봦����
| | ��һ��ʵ�� | �ڶ���ʵ�� |
| ������������g�� | 29.563 | 30.064 |
| ������������������g�� | 31.676 | 32.051 |
| ���غ�����������ͭ��������g�� | 30.911 | 31.324 |
| x��ֵ | 5.05 | 5.13 |
�����ϱ��е����ݴ�����������㱾��ʵ���������Ϊ______%����֪x������ֵΪ5����
�����������ۡ�
��1����һ��ʵ�飬������Ҫ����________�Σ������֣���ͬ����������Ҫ����_________�Ρ�
��2�����ز�����Ŀ����__________________��
��3���ظ�����ʵ����xƽ��ֵ��Ŀ����_____________________________��
��4��ʵ��ֵ������ֵƫ���ԭ�������________�����ţ���
a�����ȹ������о��彦�� b��������Ʒ�к��м��Ȳ��ӷ�������
c��ʵ��ǰ��������泱ʪ d���������պ�ֱ�ӷ��ڿ�������ȴ
ʵ���ҳ����ü�ȩ��HCHO�����ⶨ(NH4)2SO4��Ʒ�е��������������䷴Ӧԭ��Ϊ��4NH4�� ��6HCHO =3H����6H2O��(CH2)6N4H�� �۵ζ�ʱ��1 mol (CH2)6N4H���� l mol H���൱��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᡣij��ȤС���ü�ȩ������������ʵ�飺
����I ��ȡ��Ʒ1��500 g��
����II ����Ʒ�ܽ����ȫת�Ƶ�250 mL����ƿ�У����ݣ����ҡ�ȡ�
����III ��ȡ25��00 mL��Ʒ��Һ��250 mL��ƿ�У�����10 mL 20�������Լ�ȩ��Һ��ҡ�ȡ�����5 min����1~2�η�̪��Һ����NaOH����Һ�ζ����յ㡣�����������������ظ�2�Ρ�
��1�����ݲ���III��գ�
�ټ�ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ���������Ʒ�е�����������________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
����ƿ������ˮϴ�Ӻ�ˮδ��������ζ�ʱ��ȥNaOH����Һ�����_______(�ƫ����ƫС������Ӱ�족)
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�____________��
A���ζ�����Һ��ı仯 B����ƿ����Һ��ɫ�ı仯
�ܵζ��ﵽ�յ�ʱ����__________________________________________________��
��2���ζ�������±���ʾ��
| �ζ� ���� | ������Һ����� /mL | ����Һ�����/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25��00 | 1��02 | 21��03 |
| 2 | 25��00 | 2��00 | 21��99 |
| 3 | 25��00 | 0��20 | 20��20 |
��NaOH����Һ��Ũ��Ϊ0��1010 mol��L��1�������Ʒ�е�����������Ϊ___________��
�������岻��ȱ�ٵ���Ԫ�أ����뺬���Ļ�����ɲ��������������ơ����г���һ�ֳ����IJ���ҩƷ���±���˵����IJ������ݡ�
[���]ÿƬ������������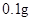 [��Ӧ֢]����ȱ����ƶѪ֢��Ԥ���������á� [�����÷�]����Ԥ���� 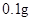 /�գ����������� /�գ�����������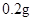 �� ��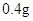 /�ա� /�ա�С������Ԥ���� 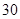 �� ��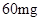 /�գ������� /�գ�������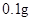 �� ��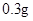 /�� /��[����]�ܹ⡢�ܷ⡢�ڸ��ﴦ���档 |
��1����ҩƷ��Fe2+�Ỻ�����������ҹ涨��ҩ����Fe2+�������ʣ��Ѿ�������Fe2+��������Fe2+�������ı�ֵ������10.00% �������ٷ��á�
��ʵ���ҿɲ���H2SO4�ữ��KMnO4��Һ���ԡ������ơ��е�Fe2+���еζ�(����ҩƷ�������ɷݲ���KMnO4��Ӧ)����д���÷�Ӧ�����ӷ���ʽ�� ��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL������ʱ��Ҫ�IJ������������������ձ�����ͷ�ι��⣬���� ��
��ijͬѧ��������еζ���ʽ���гֲ�����ȥ������������� ��������ĸ��ţ�
��2��������������Ԫ����������Ϊ20.00%�ġ������ơ�10.00 g������ȫ������ϡH2SO4�У����Ƴ�1000 ml��Һ��ȡ��20.00 ml����0.01000 mol?L-1��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00 ml ����ҩƷ��Fe2+��������Ϊ ��
��3����֪������Ϊ��Ԫ�л����ᣬ��23.6 g���������Һ��4.0 mol?L-1 100.0 ml������������Һǡ����ȫ�к͡��˴Ź�����������ʾ�������������ͼ��ֻ���������շ塣д����������Һ������������Һ��ȫ�к͵Ļ�ѧ����ʽ(�л���д�ṹ��ʽ) ��


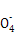 +8H+
+8H+ 5Fe3++Mn2++4H2O)��
5Fe3++Mn2++4H2O)��