��Ŀ����
Ϊ֤����ѧ��Ӧ��һ�����ȣ���������̽�����
I��ȡ5m1 0.1mol/L��KI��Һ���μӡ���FeCl3ϡ��Һ(��֪��2Fe3++2I-=I2+2Fe2+)
��������2ml CCl4��
��ȡ��ȡ����ϲ���Һ���μ�KSCN��Һ��
(1)̽���I��ʵ������Ϊ_____________________________________________
̽������ʵ������Ϊ_______________________________________________
(2)̽��������ͼ��ͨ������Ѫ��ɫ��Fe(SCN)3��Һ����֤��Fe3+�������Ӷ�֤����ѧ��Ӧ��һ�����ȣ�����ʵ����ȴδ����Һ��Ѫ��ɫ���Դ�ͬѧ��������������ֲ��룺
����һ��Fe3+ȫ��ת��ΪFe2+
����������ɵ�Fe(SCN)3Ũ�ȼ�С������ɫ�������۲졣
Ϊ����֤���룬�������ϻ��������Ϣ��
��Ϣһ����������ˮ��Fe(SCN)3�������е��ܽ�ȱ���ˮ�д�
��Ϣ����Fe3+����[Fe(CN)6] 4-��Ӧ������ɫ��������K4[Fe(CN)6](��ɫ)��Һ����Fe3+�������ȱ���KSCN���ߡ�
�������Ϣ�����������ʵ�鷽����֤���룺
��������±�
��д��ʵ����������衪"�з�Ӧ�����ӷ���ʽ��_______________________________��
����Ϊ�˲ⶨ̽���I�е�FeCl3ϡ��Һ��Ũ�ȣ��ֽ������²�����
����ȡ25.00mLFeCl3ϡ��Һ����ƿ�У�����KSCN��Һ��ָʾ��������c mol/L KI����Һ �ζ����ﵽ�ζ��յ�ʱ��������_______________________________________________��
���ظ��ζ����Σ�ƽ������c mol/L KI����ҺV mL����FeCl3ϡ��Һ���ʵ���Ũ��Ϊ________mol/L��
�����ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ����ⶨ���_______ (�ƫ�ߡ���ƫ�ͣ������䡱)��
I��ȡ5m1 0.1mol/L��KI��Һ���μӡ���FeCl3ϡ��Һ(��֪��2Fe3++2I-=I2+2Fe2+)
��������2ml CCl4��
��ȡ��ȡ����ϲ���Һ���μ�KSCN��Һ��
(1)̽���I��ʵ������Ϊ_____________________________________________
̽������ʵ������Ϊ_______________________________________________
(2)̽��������ͼ��ͨ������Ѫ��ɫ��Fe(SCN)3��Һ����֤��Fe3+�������Ӷ�֤����ѧ��Ӧ��һ�����ȣ�����ʵ����ȴδ����Һ��Ѫ��ɫ���Դ�ͬѧ��������������ֲ��룺
����һ��Fe3+ȫ��ת��ΪFe2+
����������ɵ�Fe(SCN)3Ũ�ȼ�С������ɫ�������۲졣
Ϊ����֤���룬�������ϻ��������Ϣ��
��Ϣһ����������ˮ��Fe(SCN)3�������е��ܽ�ȱ���ˮ�д�
��Ϣ����Fe3+����[Fe(CN)6] 4-��Ӧ������ɫ��������K4[Fe(CN)6](��ɫ)��Һ����Fe3+�������ȱ���KSCN���ߡ�
�������Ϣ�����������ʵ�鷽����֤���룺
��������±�
| ʵ����� | ����ͽ��� |
| ����һ��ȡ��ȡ����ϲ���Һ�μ�2��K4[Fe(CN)6] | ��_________�������һ�������� |
| ���������̽����������Һ�м��������� �ѣ���������÷ֲ� | �����Ѳ��Ѫ��ɫ����___________�� |
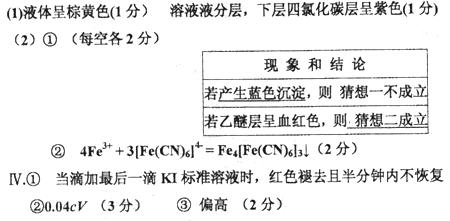
����Ϊ�˲ⶨ̽���I�е�FeCl3ϡ��Һ��Ũ�ȣ��ֽ������²�����
����ȡ25.00mLFeCl3ϡ��Һ����ƿ�У�����KSCN��Һ��ָʾ��������c mol/L KI����Һ �ζ����ﵽ�ζ��յ�ʱ��������_______________________________________________��
���ظ��ζ����Σ�ƽ������c mol/L KI����ҺV mL����FeCl3ϡ��Һ���ʵ���Ũ��Ϊ________mol/L��
�����ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ����ⶨ���_______ (�ƫ�ߡ���ƫ�ͣ������䡱)��
��

��ϰ��ϵ�д�
�����Ŀ
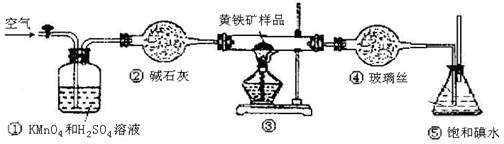
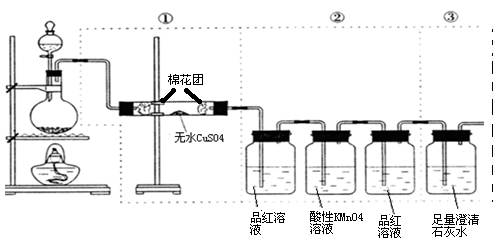

 ���Լ�Y�����ƣ�
���Լ�Y�����ƣ� 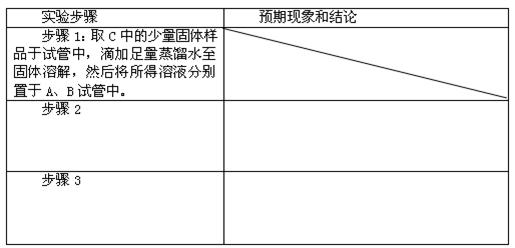

 ���� ��˵����Ӧ���ȡ�������װ����֧�ż��������������ȥ��
���� ��˵����Ӧ���ȡ�������װ����֧�ż��������������ȥ��
 ��
��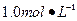 Ba(OH)2��Һ����ֻ�ҵ��ڿ����б�¶�Ѿõ�Ba(OH)2��8H2O�Լ�����ѧʽ����315������������������Һʱ������ȡ�Լ���ˮ�н������ܽ⣬�ձ��д��ڴ���δ���Ϊ̽��ԭ��ͬѧ���Ba(OH)2��8H2O��283K��293K��303Kʱ���ܽ�ȣ�g/100g H2O���ֱ�Ϊ2.5��3.9��5.6��
Ba(OH)2��Һ����ֻ�ҵ��ڿ����б�¶�Ѿõ�Ba(OH)2��8H2O�Լ�����ѧʽ����315������������������Һʱ������ȡ�Լ���ˮ�н������ܽ⣬�ձ��д��ڴ���δ���Ϊ̽��ԭ��ͬѧ���Ba(OH)2��8H2O��283K��293K��303Kʱ���ܽ�ȣ�g/100g H2O���ֱ�Ϊ2.5��3.9��5.6��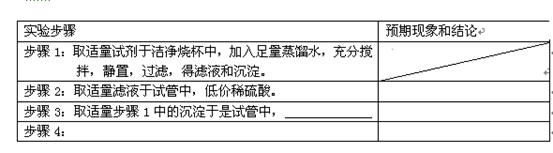
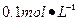 Ba(OH)2��8H2O��Һ��ȷ��ȡw�������������ձ��У�����������ˮ�� ������Һת�� ��ϴ�ӣ����ݣ�ҡ�ȡ�
Ba(OH)2��8H2O��Һ��ȷ��ȡw�������������ձ��У�����������ˮ�� ������Һת�� ��ϴ�ӣ����ݣ�ҡ�ȡ� ����װ��50ml��ʽ�ζ��ܣ��ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ�ƽ����������Vml��
����װ��50ml��ʽ�ζ��ܣ��ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ�ƽ����������Vml��