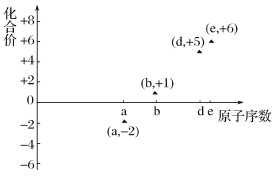
��Ŀ����
����Ŀ�����Ǻϳ����ᡢ��κ͵��ʵĻ���ԭ�ϣ��ش��������⣺
��1��NH3�ĵ���ʽ______��
��2���Ȼ��ˮ��Һ�����ԣ���ԭ��Ϊ______�������ӷ���ʽ��ʾ����0.1mol/L�İ�ˮ�м���������NH4Cl���壬��Һ��pH______������������������������������������������������볢�Դ�ƽ���ƶ��ĽǶȽ�����Һ��NH4+Ũ�ȵı仯ԭ��______��
��3������識��ȷֽ�ɵõ�N2O��g����H2O��g����250��ʱ����������ܱ������зֽ�ﵽƽ�⣬���¶��·�Ӧ��ƽ�ⳣ������ʽΪ______������1mol�������ȫ�ֽ⣬ת�Ƶĵ�����Ϊ______mol��
��4��3H2��g��+N2��g��2NH3��g����H=-92kJ/mol������Ӧ�ų�9.2kJ ��������μӷ�Ӧ������������ĿΪ______��
���𰸡�![]() NH4++H2ONH3H2O+H+ ���� ���������������������������������ӷ�Ӧ���ٽ�һˮ�ϰ��ĵ��룬��Һ�е�NH4+��Ũ������ K=c��N2O����c2��H2O�� 4 0.3NA
NH4++H2ONH3H2O+H+ ���� ���������������������������������ӷ�Ӧ���ٽ�һˮ�ϰ��ĵ��룬��Һ�е�NH4+��Ũ������ K=c��N2O����c2��H2O�� 4 0.3NA
��������
��1������ԭ�Ӻ���ԭ�ӹ��õ��ӶԵ����дNH3�ĵ���ʽ��
��2���Ȼ��ˮ��Һ��ˮ�������ԣ��ݴ�д���ӷ���ʽ��0.1mol/L�İ�ˮ�м���������NH4Cl���壬��ͬ����ЧӦ�Ե���ƽ���Ӱ���ж���Һ��pH�仯��������������������������������Ӷ�ˮ����ƽ���Ӱ���ж�NH4+Ũ�ȵı仯��ԭ��
��3��������識��ȷֽ�ɵõ�N2O(g)��H2O(g)��д250��ʱ����������ܱ������зֽ�Ļ�ѧ����ʽ���ݴ�д���¶��·�Ӧ��ƽ�ⳣ������ʽ��������立ֽⷽ��ʽ�����������ӵ����ʵ����Ĺ�ϵ������ת�Ƶĵ�������
��4����3H2(g)+N2(g)2NH3(g)��H=-92kJ/mol��ʵ�����ĵ����������ʵ�����ų������Ĺ�ϵ���ɼ���ų�9.2kJ ����ʱ�μӷ�Ӧ������������Ŀ��
��1�������ǹ��ۻ����Nԭ�Ӻ�Hԭ��֮���Թ��ۼ����ϣ��������ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��2���Ȼ�淋�ˮ��Һ�������ԣ���ԭ��ΪNH4++H2ONH3H2O+H+��0.1molL-1�İ�ˮ�м���������NH4Cl���壬�����Ũ����������һˮ�ϰ��ĵ��룬����������Ũ�ȼ�С����Һ��pH���ͣ������������������������������������ӷ�Ӧ���ٽ�һˮ�ϰ��ĵ��룬��Һ�е�NH4+��Ũ������
�ʴ�Ϊ��NH4++H2ONH3H2O+H+�� ���ͣ� ���������������������������������ӷ�Ӧ���ٽ�һˮ�ϰ��ĵ��룬��Һ�е�NH4+��Ũ������
��3������立ֽ�����N2O��H2O���ﵽƽ�⣬˵��Ϊ���淴Ӧ����ѧ��Ӧ����ʽΪ��NH4NO3![]() N2O+2H2O��250��ʱ��ˮΪ����״̬����ƽ�ⳣ��K=c(N2O)��c2(H2O)��NH4NO3��NH4+��NԪ�ػ��ϼ�Ϊ-3�ۣ�NO3-�е�NԪ�صĻ��ϼ�Ϊ+5�ۣ���Ӧ��NԪ�صĻ��ϼ�Ϊ+1�ۣ��������з�Ӧ��NԪ����-3������Ϊ+1�ۣ��˷�Ӧ��ÿ�ֽ�1mol����泥�ת�Ƶ�����Ϊ4mol��
N2O+2H2O��250��ʱ��ˮΪ����״̬����ƽ�ⳣ��K=c(N2O)��c2(H2O)��NH4NO3��NH4+��NԪ�ػ��ϼ�Ϊ-3�ۣ�NO3-�е�NԪ�صĻ��ϼ�Ϊ+5�ۣ���Ӧ��NԪ�صĻ��ϼ�Ϊ+1�ۣ��������з�Ӧ��NԪ����-3������Ϊ+1�ۣ��˷�Ӧ��ÿ�ֽ�1mol����泥�ת�Ƶ�����Ϊ4mol��
�ʴ�Ϊ��K= c(N2O)��c2(H2O)��4��
��4��3H2(g)+N2(g)2NH3(g)��H=-92kJ/mol����֪�ų�92kJ����ʱ����������Ϊ3mol���ʵ���Ӧ�ų�9.2kJ ��������μӷ�Ӧ������Ϊ0.3mol��������Ϊ0.3NA��
�ʴ�Ϊ��0.3NA��

����Ŀ��ú̿ȼ��ʱ��������SO2��NO�Ի���Ӱ�켫��
(1)ʹ�������Դ����Ч����SO2�ȵ��ŷš�ú��Һ�����ִ���Դ��ҵ���ص��ƹ����Դ�ۺ����÷����������Һ������Ϊ��ú����CH3OH����֪�Ʊ��״����йػ�ѧ��Ӧ��ƽ�ⳣ�����£�
i��CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)����H1����a kJ/mol
CH3OH(g)��H2O(g)����H1����a kJ/mol
ii��CO2(g)��H2(g) ![]() CO(g)��H2O(g) ��H2����b kJ/mol
CO(g)��H2O(g) ��H2����b kJ/mol
iii��CO(g)��2H2(g) ![]() CH3OH(g)����H3
CH3OH(g)����H3
��H3��________��
(2)���ܱ������н��з�Ӧi���ı��¶�ʱ���÷�Ӧ�е��������ʶ�Ϊ��̬����ʼ�¶ȡ������ͬ(T1 �桢2 L�ܱ�����)����Ӧ�����в������ݼ��±���
��Ӧʱ�� | CO2/mol | H2/mol | CH3OH/mol | H2O/mol | |
��Ӧ��� �º��� | 0 min | 2 | 6 | 0 | 0 |
10 min | 4.5 | ||||
20 min | 1 | ||||
30 min | 1 | ||||
��Ӧ��� �Ⱥ��� | 0 min | 0 | 0 | 2 | 2 |
�ٴﵽƽ���Ӧ��Աȣ�ƽ�ⳣ��K(��)________K(��)(�>����<��������ͬ)��ƽ��ʱCO2��Ũ��c(��)________c(��)��
�ڶԷ�Ӧ�����������������£���30 minʱֻ�ı��¶�ΪT2 �棬�ٴ�ƽ��ʱH2�����ʵ���Ϊ2.5 mol����T1________T2(�>����<������)��
����30 minʱֻ���������ٳ���1 mol H2(g)��1 mol H2O(g)����ƽ��________�ƶ�(�����������)��
(3)�о���Ա���֣���ú̿��O2/CO2��������ȼ�գ��ܹ�����ȼúʱNO���ŷţ���Ҫ��ӦΪ��2NO(g)��2CO(g)=N2(g)��2CO2(g)����һ���¶��£���2 L�ĺ����ܱ������г���0.1 mol NO��0.3 mol CO�����÷�Ӧ����ͼΪ�����ڵ�ѹǿ(p)����ʼѹǿ(p0)�ı�ֵ(p/p0)��ʱ��ı仯���ߡ�
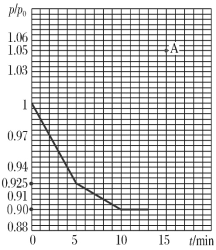
��0��5 min�ڣ��÷�Ӧ��ƽ����Ӧ����v(NO)��________��ƽ��ʱN2�IJ���Ϊ________��
����13 minʱ������������ٳ���0.06 mol CO2��15 minʱ�ٴδﵽƽ�⣬��ʱ������![]() /
/![]() �ı�ֵӦ��ͼ��A���________(��Ϸ������·���)��
�ı�ֵӦ��ͼ��A���________(��Ϸ������·���)��