��Ŀ����
4����1��ij����A���������ܶ�����ͬ״���������ܶȵ�64�������ⶨ��֪A�����й�����6����������A����ϩ���������ӳɵIJ����A������Ϊ��2��2��4��4-�ļ����飻
����A��Ȳ���������ӳɵIJ����A�Ľṹ��ʽΪ��CH3CH2C��CH3��2C��CH3��3��
��2��ij��A 0.1mol �������г��ȼ�պ�������22gCO2��9gH2O���Իش��������⣺
������A����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ��ȡ���������֣�����A�ĽṹΪ
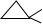 ��
��������A��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���3����������A���ܵĽṹ��ʽCH2=CHCH��CH3��2��CH3CH=C��CH3��2��CH3CH2C��CH3��=CH2�����ƣ�3-��-1-��ϩ��2-��-2-��ϩ��2-��-1-��ϩ��
���� ��1������A�����ܶ�����ͬ״���������ܶȵ�64������Mr��A��=1��64=128����A�����ΪCnH2n+2����12n+2n+2=128�����n=9����������A�ķ���ʽΪC9H20��A�����й�����6������
����A����ϩ���������ӳɵIJ��˵������2��Cԭ���ϲ����ܶ�������ԭ�ӣ�A�Ľṹ��ʽΪ����CH3��3CCH2C��CH3��3��
����A��Ȳ���ļӳɲ��˵������2��Cԭ���Ͼ�������2����ԭ�ӣ�
��2��22gCO2�����ʵ���Ϊ$\frac{22g}{44g/mol}$=0.5mol��9gH2O�����ʵ���Ϊ$\frac{9g}{18g/mol}$=0.5mol����A������N��C��=$\frac{0.5mol}{0.1mol}$=5��N��H��=$\frac{0.5mol��2}{0.1mol}$=10����A�ķ���ʽΪC5H10��
������A����ʹ��ˮ��ɫ�����������ͼ���Ӧ����1����״�ṹ������һ��������������������ȡ����Ӧ����һ��ȡ���������֣���������2��Hԭ�ӣ�Ӧ����1����Ԫ������ͬһ̼ԭ������2������
������A��ʹ��ˮ��ɫ������̼̼˫������H2�ӳɲ�������к���3�������ӳɲ���ΪCH3CH2CH��CH3��2����ԭ̼̼˫���жϿ��ܵĽṹ��ʽ������������
��� �⣺��1������A�����ܶ�����ͬ״���������ܶȵ�64������Mr��A��=1��64=128����A�����ΪCnH2n+2����12n+2n+2=128�����n=9����������A�ķ���ʽΪC9H20��A�����й�����6������
����A����ϩ���������ӳɵIJ��˵������2��Cԭ���ϲ����ܶ�������ԭ�ӣ�A�Ľṹ��ʽΪ����CH3��3CCH2C��CH3��3������Ϊ��2��2��4��4-�ļ����飬
�ʴ�Ϊ��2��2��4��4-�ļ����飻
����A��Ȳ���ļӳɲ��˵������2��Cԭ���Ͼ�������2����ԭ�ӣ���A�Ľṹ��ʽΪ��CH3CH2C��CH3��2C��CH3��3��
�ʴ�Ϊ��CH3CH2C��CH3��2C��CH3��3��
��2��22gCO2�����ʵ���Ϊ$\frac{22g}{44g/mol}$=0.5mol��9gH2O�����ʵ���Ϊ$\frac{9g}{18g/mol}$=0.5mol����A������N��C��=$\frac{0.5mol}{0.1mol}$=5��N��H��=$\frac{0.5mol��2}{0.1mol}$=10����A�ķ���ʽΪC5H10��
������A����ʹ��ˮ��ɫ�����������ͼ���Ӧ����1����״�ṹ������һ��������������������ȡ����Ӧ����һ��ȡ���������֣���������2��Hԭ�ӣ�Ӧ����1����Ԫ������ͬһ̼ԭ������2�������ṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
������A��ʹ��ˮ��ɫ������̼̼˫������H2�ӳɲ�������к���3�������ӳɲ���ΪCH3CH2CH��CH3��2����ԭ̼̼˫����֪�����ܵĽṹ��ʽ�У�CH2=CHCH��CH3��2��CH3CH=C��CH3��2��CH3CH2C��CH3��=CH2�����Ʒֱ�Ϊ��3-��-1-��ϩ��2-��-2-��ϩ��2-��-1-��ϩ��
�ʴ�Ϊ��CH2=CHCH��CH3��2��CH3CH=C��CH3��2��CH3CH2C��CH3��=CH2��3-��-1-��ϩ��2-��-2-��ϩ��2-��-1-��ϩ��
���� ���⿼���л������ʽ��ȷ������������ͬ���칹�����д���ϺõĿ���ѧ�����������������ṹ��ʽ����дΪ�״��㣬�Ѷ��еȣ�

| A�� | Ũ������������Na+ | B�� | ��Һ������ | ||
| C�� | c��K+��=0.05mol/L | D�� | ������������������� |
�����ѣ�Ti������Ӳ�ȴ��۵�ߡ�����ʱ����ʴ�����㷺�������¿Ƽ����ϣ�����Ϊ��δ��������������������Ҫ�ɷ�FeTiO3������������Ϊ��Ҫԭ��ұ��������ͬʱ��ø���Ʒ�Ĺ�ҵ�����������£�
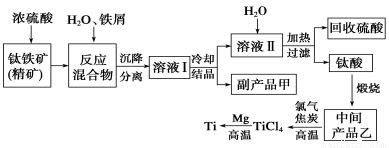
�ش��������⣺
��1���������Ũ���ᷴӦ�IJ���֮һ��TiOSO4����Ӧ�����������ɡ�����Ʒ����������________��
��2���������������м�����м��Ŀ����____________________________��
��3����ʱ��Һ���к���Fe2����TiO2��������Mg2���������ӡ������£����Ӧ���������Ksp���±���ʾ��
�������� | Fe��OH��2 | TiO��OH��2 | Mg��OH��2 |
Ksp | 8��0��10��16 | 1��0��10��29 | 1��8��10��11 |
�ٳ����£���������Һ��Mg2�������ʵ���Ũ��Ϊ0��0018mol��L��1������Һ��pH����________ʱ��Mg��OH��2��ʼ������
����������Fe2����TiO2����Mg2������Һ��ˮϡ�ͣ���������������ɫ������д���÷�Ӧ�����ӷ���ʽ��________________________��
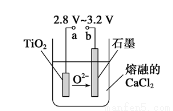
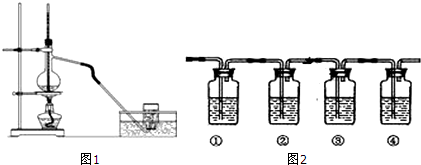
��4��Mg��ԭTiCl4�����б�����1070K���¶��½��У�����Ϊ��Ӧ�ÿ��Ƶķ�Ӧ������__________________����5����800��1000��ʱ���TiO2Ҳ���Ƶú����ѣ�װ����ͼ��ʾ����ͼ��b�ǵ�Դ��________���������ĵ缫��Ӧʽ________��
| A�� | ȫ�� | B�� | ֻ�Т� | C�� | �ں͢� | D�� | �ڢۢ� |
| A�� | 2�� | B�� | 4�� | C�� | 6�� | D�� | 8�� |
| A�� | ����CsClʱ���������������ɫ | |
| B�� | CsNO3������ˮ | |
| C�� | ���ơ��ء�����ֵ����У��Ƶ��۵���� | |
| D�� | CsOH������ |
 һ���¶��£��ں����ܱ������г���2molNO2��1molO2������Ӧ���£�4NO2��g��+O2��g��?2N2O5��g��
һ���¶��£��ں����ܱ������г���2molNO2��1molO2������Ӧ���£�4NO2��g��+O2��g��?2N2O5��g����1����֪ƽ�ⳣ��K350����K300������÷�Ӧ�Ƿ��ȷ�Ӧ������ȡ����ȡ����� �����£��÷�Ӧ�������Է����У�ԭ�����淴Ӧ����ġ�S��0��
��2�������йظ÷�Ӧ��˵����ȷ����BD
A���������������ƽ�����淴Ӧ�����ƶ������������ɫ����
B�����º����£��ٳ���2molNO2��1molO2���ٴδﵽƽ��ʱNO2ת��������
C�����º����£��������ڵ��ܶȲ��ٸı䣬��Ӧ�ﵽƽ��״̬
D�����÷�Ӧ��ƽ�ⳣ��������һ���ǽ������¶�
��3�����Ļ���������϶࣬��NH3��NO��NO2��HNO3�������εȣ�
����������һ�����ᣬ��֤����������������ʵ���AD
A�������£�����������Һ��pH��7
B���������ܺ�NaOH�����кͷ�Ӧ
C�������� �� ��Һ��������ʵ�飬���ݺܰ�
D�������£���pH=3����������Һϡ��10����pH��4
�ڸ�������������ۣ������ܸ������ӵķ��ӻ����Ӷ����ᣬ�����ܽ�����ӵķ��ӻ����Ӷ��Ǽ����������ۣ������������������ʵ���acd
a��H2O b��NO2- c��H2NCH2COOH d��H2PO4- e��H2S
�۵�ͬ������Ԫ���γɵ�Na2HPO4��Һ�Լ��ԣ�������Һ�м���������CaCl2��Һ����Һ�������ԣ���ԭ����3Ca2++2HPO42-�TCa3��PO4��2��+2H+�������ӷ���ʽ��ʾ����
��4��X��Y��Z��W�ֱ���HNO3��NH4NO3��NaOH��NaNO2����ǿ������е�һ�֣��±��dz�����Ũ�Ⱦ�Ϊ0.01mol•L-1��X��Y��Z��W��Һ��pH��
| 0.01mol•L-1��Һ | X | Y | Z | W |
| pH | 12 | 2 | 8.5 | 4.5 |
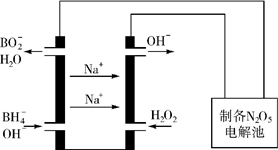
��5��N2O5��һ��������ɫ�����������Ʊ����������⻯��ȼ�ϵ������Դ�����õ�ⷨ�Ʊ��õ�N2O5������ԭ����ͼ�������⻯��ȼ�ϵ�صĸ�����ӦʽΪBH4-+8OH--8e-=BO2-+6H2O��
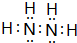 ������ԭ�Ӵ���ͬһƽ���𣿷��ǣ�����
������ԭ�Ӵ���ͬһƽ���𣿷��ǣ�����