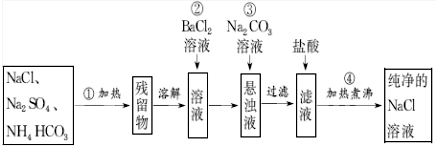
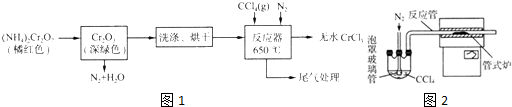
��Ŀ����
12��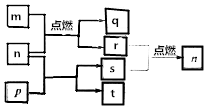 ����������Ԫ��X��Y��Z��Wԭ��������������m��W�ĵ��ʣ�r��X�ĵ��ʣ�s��Y�ĵ��ʣ�n��p��q����ЩԪ����ɵĶ�Ԫ�����t�����е�����Ԫ����ɣ���ˮ��Һ�ʼ��ԣ�p�ǵ���ɫ���壬q��һ�����²��ϣ����ǵĹ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
����������Ԫ��X��Y��Z��Wԭ��������������m��W�ĵ��ʣ�r��X�ĵ��ʣ�s��Y�ĵ��ʣ�n��p��q����ЩԪ����ɵĶ�Ԫ�����t�����е�����Ԫ����ɣ���ˮ��Һ�ʼ��ԣ�p�ǵ���ɫ���壬q��һ�����²��ϣ����ǵĹ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | Y��Z��W�ļ����ӵĵ��Ӳ�ṹ��ͬ | |
| B�� | ԭ�Ӱ뾶��r��X����r��Y����r��Z����r��W�� | |
| C�� | ���⻯����ȶ��ԣ�X��Y | |
| D�� | Y��Z����Ԫ���γɵĻ�����һ��ֻ�����Ӽ� |
���� ����������Ԫ��X��Y��Z��Wԭ��������������m��W�ĵ��ʣ�r��X�ĵ��ʣ�s��Y�ĵ��ʣ�n��p��q����ЩԪ����ɵĶ�Ԫ�����t�����е�����Ԫ����ɣ���ˮ��Һ�ʼ��ԣ�p�ǵ���ɫ���壬q��һ�����²��ϣ����ͼת����֪��pΪNa2O2��nΪCO2��tΪNa2CO3��sΪO2��mΪMg��qΪMgO��rΪC����Ԫ��XΪC��YΪO��ZΪNa��WΪMg��Ȼ����Ԫ�������������
��� �⣺����������Ԫ��X��Y��Z��Wԭ��������������m��W�ĵ��ʣ�r��X�ĵ��ʣ�s��Y�ĵ��ʣ�n��p��q����ЩԪ����ɵĶ�Ԫ�����t�����е�����Ԫ����ɣ���ˮ��Һ�ʼ��ԣ�p�ǵ���ɫ���壬q��һ�����²��ϣ����ͼת����֪��pΪNa2O2��nΪCO2��tΪNa2CO3��sΪO2��mΪMg��qΪMgO��rΪC����Ԫ��XΪC��YΪO��ZΪNa��WΪMg��
A��Y��Z��W�ļ����ӵĵ��Ӳ�ṹ��ͬ����Ϊ10���ӽṹ����A��ȷ��
B�����Ӳ�Խ�࣬ԭ�Ӱ뾶Խ��ͬ����ԭ���������ԭ�Ӱ뾶С����ԭ�Ӱ뾶��r��Y����r��X����r��W����r��Z������B����
C���ǽ�����Y��X������⻯����ȶ��ԣ�X��Y����C����
D��Y��Z����Ԫ���γɵĻ�����ΪNa2O�������Ӽ�����ΪNa2O2ʱ�����Ӽ����ۼ�����D����
��ѡA��
���� ���⿼��������ƶϣ�Ϊ��Ƶ���㣬����ͼ��ת����Ӧ��pΪ��������Ϊ���Ĺؼ������ط������ƶ������Ŀ��飬ע��Ԫ�ػ�����֪ʶ��Ԫ�������ɵ�Ӧ�ã���Ŀ�ѶȲ���

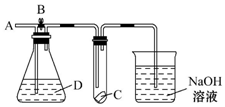 ��ͼ��ʾ��A��ͨ������Cl2����B����C���ĺ첼������ɫ���ر�B��ʱ��C���ĺ첼������������������Dƿ��װ���ǣ�������
��ͼ��ʾ��A��ͨ������Cl2����B����C���ĺ첼������ɫ���ر�B��ʱ��C���ĺ첼������������������Dƿ��װ���ǣ�������| A�� | Ũ���� | B�� | NaOH��Һ | C�� | Ũ���� | D�� | ����NaCl��Һ |
| A�� | Na2O2�ڷ�Ӧ��ֻ�������� | |
| B�� | ����Na2O2��H2O��Ӧ�ų�������������������֬����ȼ�� | |
| C�� | Na2O2��H2O��Ӧ���������������ɣ�Na2O2�ǵ��͵ļ��������� | |
| D�� | Na2O2��H2O��Ӧ�����������ɣ������ڷ�������� |
| A�� | 5.1 g | B�� | 10.2 g | C�� | 13.6 g | D�� | 15.3 g |
| A�� | 0.308 | B�� | 308 | C�� | 154 | D�� | 0.154 |