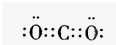��Ŀ����
������Ԫ��A��B��C��D��Eԭ���������ε�����Aԭ�������������Ǵ�����������2����B�ĵ���Ϊ˫ԭ�ӷ��ӣ����⻯����ʹʪ���ʯ����ֽ������C��ͬ����Ԫ����ԭ�Ӱ뾶���D���������������ڵ��Ӳ�����E�����������+7�ۣ�
��1��д��EԪ�������ڱ��е�λ��
��2��д��AԪ����̬�⻯��Ľṹʽ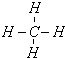
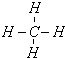
��3��B���⻯���E���⻯�ﷴӦ����������ĵ���ʽΪ
 ��
��
��4��D���ʺ�C������������Ӧ��ˮ�������Һ��Ӧ�����ӷ���ʽΪ
��5��A������B������������Ӧ��ˮ�����Ũ��Һ��Ӧ�Ļ�ѧ����ʽΪ
��1��д��EԪ�������ڱ��е�λ��
�������ڵ�VIIA��
�������ڵ�VIIA��
��2��д��AԪ����̬�⻯��Ľṹʽ
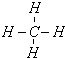
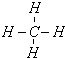
��3��B���⻯���E���⻯�ﷴӦ����������ĵ���ʽΪ


��4��D���ʺ�C������������Ӧ��ˮ�������Һ��Ӧ�����ӷ���ʽΪ
2Al+2OH-+2H2O�T2AlO2-+3H2��
2Al+2OH-+2H2O�T2AlO2-+3H2��
��5��A������B������������Ӧ��ˮ�����Ũ��Һ��Ӧ�Ļ�ѧ����ʽΪ
C+4HNO3��Ũ��
CO2��+4NO2��+2H2O
| ||
C+4HNO3��Ũ��
CO2��+4NO2��+2H2O
��
| ||
������������Ԫ��A��B��C��D��Eԭ���������ε�����Aԭ�������������Ǵ�����������2������A��������Ϊ6����AΪC��B�ĵ���Ϊ˫ԭ�ӷ��ӣ����⻯����ʹʪ���ʯ����ֽ��������BΪN��C��ͬ����Ԫ����ԭ�Ӱ뾶���C�ڵ������ڣ���CΪNa��D���������������ڵ��Ӳ�������DΪ�������ڵڢ�A��Ԫ�أ���DΪAl��E�����������+7�ۣ���E�ڵ������ڵڢ���A�壬��EΪCl��Ȼ������Ԫ�ؼ��䵥�ʡ�����������������
����⣺������Ԫ��A��B��C��D��Eԭ���������ε�����Aԭ�������������Ǵ�����������2������A��������Ϊ6����AΪC��B�ĵ���Ϊ˫ԭ�ӷ��ӣ����⻯����ʹʪ���ʯ����ֽ��������BΪN��C��ͬ����Ԫ����ԭ�Ӱ뾶���C�ڵ������ڣ���CΪNa��D���������������ڵ��Ӳ�������DΪ�������ڵڢ�A��Ԫ�أ���DΪAl��E�����������+7�ۣ���E�ڵ������ڵڢ���A�壬��EΪCl��
��1��EΪCl��������Ϊ17���ڵ������ڵ�VIIA�壬�ʴ�Ϊ���������ڵ�VIIA�壻��
��2��AΪC���⻯��Ϊ���飬C��H֮���γɹ��ۼ�����ṹʽΪ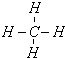 ���ʴ�Ϊ��
���ʴ�Ϊ��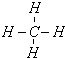 ��
��
��3��B���⻯��Ϊ������E���⻯��ΪHCl�����߷�Ӧ�����Ȼ�泥�Ϊ���ӻ��������ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4��D����ΪAl��C������������Ӧ��ˮ����ΪNaOH�����߷�Ӧ�����ӷ�ӦΪ2Al+2OH-+2H2O�T2AlO2-+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O�T2AlO2-+3H2����
��5��B������������Ӧ��ˮ�����Ũ��ҺΪŨ���ᣬ
C��Ũ����ķ�ӦΪC+4HNO3��Ũ��
CO2��+4NO2��+2H2O��
�ʴ�Ϊ��C+4HNO3��Ũ��
CO2��+4NO2��+2H2O��
��1��EΪCl��������Ϊ17���ڵ������ڵ�VIIA�壬�ʴ�Ϊ���������ڵ�VIIA�壻��
��2��AΪC���⻯��Ϊ���飬C��H֮���γɹ��ۼ�����ṹʽΪ
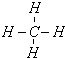 ���ʴ�Ϊ��
���ʴ�Ϊ��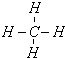 ��
����3��B���⻯��Ϊ������E���⻯��ΪHCl�����߷�Ӧ�����Ȼ�泥�Ϊ���ӻ��������ʽΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����4��D����ΪAl��C������������Ӧ��ˮ����ΪNaOH�����߷�Ӧ�����ӷ�ӦΪ2Al+2OH-+2H2O�T2AlO2-+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O�T2AlO2-+3H2����
��5��B������������Ӧ��ˮ�����Ũ��ҺΪŨ���ᣬ
C��Ũ����ķ�ӦΪC+4HNO3��Ũ��
| ||
�ʴ�Ϊ��C+4HNO3��Ũ��
| ||
���������⿼��ԭ�ӽṹ��Ԫ�������ɣ���Ϥԭ�ӽṹ��Ԫ�ص������ǽ����Ĺؼ����ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ