��Ŀ����
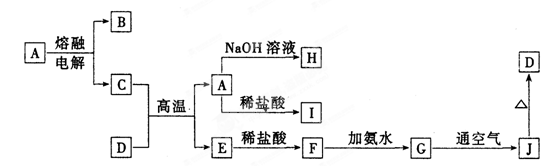
��12�֣�A��J����ѧ��ѧ���������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������֪A��һ�ָ��۵����ʣ�D��һ�ֺ���ɫ���塣
 ��ش�
��ش�
�������⣺
��1��A���ʵ�����Ϊ_________��H��I��Ӧ�Ļ�ѧ����ʽ
��2��C��D�ڸ����µķ�Ӧ��ұ��ҵ�ϳ�Ϊ ��Ӧ�������÷�Ӧ��ʵ�������
___
��3��д��G��J�Ļ�ѧ����ʽ��___ ��
��4��A��H�����ӷ���ʽΪ___________________________________________
��5�������ӷ���ʽ��ʾI���������ھ�ˮ��ԭ��_____________________ _
 ��ش�
��ش��������⣺
��1��A���ʵ�����Ϊ_________��H��I��Ӧ�Ļ�ѧ����ʽ
��2��C��D�ڸ����µķ�Ӧ��ұ��ҵ�ϳ�Ϊ ��Ӧ�������÷�Ӧ��ʵ�������
___
��3��д��G��J�Ļ�ѧ����ʽ��___ ��
��4��A��H�����ӷ���ʽΪ___________________________________________
��5�������ӷ���ʽ��ʾI���������ھ�ˮ��ԭ��_____________________ _
��1����������1�֣� 3NaAlO2+AlCl3+6H2O=4Al(OH)3+3NaCl��2�֣�
��2�����ȣ�1�֣� ������KClO3������þ���������ȼ��2�֣�
��3��4Fe(OH)2+O2+2H2O=4Al(OH)3��2�֣�
��4��Al2O3+2OH-+H2O=2AlO2-+H2O��2�֣�
��5��Al3++3H2O Al(OH)3(����)+3H+��2�֣�
Al(OH)3(����)+3H+��2�֣�
��2�����ȣ�1�֣� ������KClO3������þ���������ȼ��2�֣�
��3��4Fe(OH)2+O2+2H2O=4Al(OH)3��2�֣�
��4��Al2O3+2OH-+H2O=2AlO2-+H2O��2�֣�
��5��Al3++3H2O
 Al(OH)3(����)+3H+��2�֣�
Al(OH)3(����)+3H+��2�֣�����������ͼ�⣬��������ͻ�Ƶ㡣D��һ�ֺ���ɫ���壬����D����������J������������G������������������E������F���Ȼ�������A��һ�ָ��۵����ʣ���A��������������B��������C������H��ƫ�����ƣ�I���Ȼ���.

��ϰ��ϵ�д�
�����Ŀ

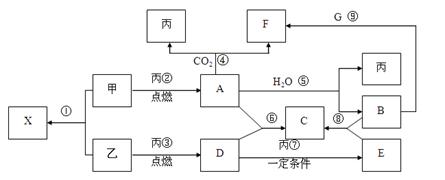
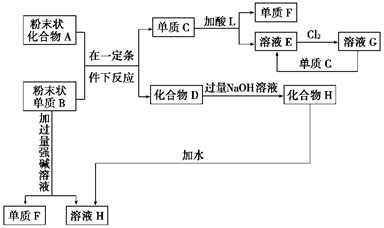
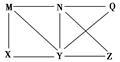

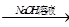 ��
�� ��
�� �ף���ײ�������
�ף���ײ�������