��Ŀ����
1����1���£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϣ���֪N2H4����������ȫȼ���е������ɣ�N2H4��ȫȼ�շ�Ӧ�Ļ�ѧ����ʽΪN2H4+O2=N2+2H2O����2��ͼ1��һ���绯ѧ����ʾ��ͼ��
��пƬ�Ϸ����ĵ缫��Ӧ��Cu2++2e-=Cu��
�ڼ���ʹ����-����ȼ�ϵ����Ϊ�������еĵ�Դ��ͭƬ�������仯128g������һ����ȼ�ϵ�����������ı�״���µĿ���112L����������������������Ϊ20%����
��3����ͳ�Ʊ��µķ���������NaClO����NH3���Ƶ��µ�ϡ��Һ���÷�Ӧ�����ӷ���ʽ��ClO-+2NH3=N2H4+Cl-+H2O��
��4��1998��ϣ������˹��´�ѧ��Marnellos��Stoukides���ø����ӵ����Ե�SCY�մɣ��ܴ���H+����ʵ���˸��³�ѹ�¸�ת���ʵĵ�ⷨ�ϳɰ�����ʵ��װ����ͼ2����ͨ��N2��һ��Ӧ�����ֱ����ĸ���������

���� ��1������N2H4����������ȫȼ�����ɵ�����H2O��д������Ӧ�Ļ�ѧ����ʽ��
��2����пΪ������ͭ���ӵõ�������ͭ��
��ͭƬ�������仯128g��n��Cu��=2mol����������ʽΪCu2++2e-=Cu����һ����ȼ�ϵ���ڼ��Ի����µ�������ӦʽΪ��2H2O+O2+4e-=4OH-�����2Cu��O2���㣻
��3��NaClO����NH3�����Ƶ��£��������Ȼ��ƣ�
��4���ϳɰ���Ӧ�е�������ԭ����ӦΪ���ص�������
��� �⣺��1����ΪN2H4����������ȫȼ�����ɵ�����H2O�������仯ѧ��Ӧ����ʽΪ��N2H4+O2=N2+2H2O��
�ʴ�Ϊ��N2H4+O2=N2+2H2O��
��2����пΪ������ͭ���ӵõ�������ͭ���缫����ʽΪCu2++2e-=Cu���ʴ�Ϊ��Cu2++2e-=Cu��
��ͭƬ�������仯128g��n��Cu��=2mol����������ʽΪCu2++2e-=Cu����ת��4mol���ӣ���һ����ȼ�ϵ���ڼ��Ի����µ�������ӦʽΪ��2H2O+O2+4e-=4OH-��������1mol���������Ϊ22.4L�������������������Ϊ20%�������Ŀ��������Ϊ$\frac{22.4L}{20%}$=112L��
�ʴ�Ϊ��112��
��3��NaClO����NH3�����Ƶ��µ����ӷ���ʽΪ��ClO-+2NH3=N2H4+Cl-+H2O��
�ʴ�Ϊ��ClO-+2NH3=N2H4+Cl-+H2O��
��4���ϳɰ���Ӧ�е�������ԭ����ӦΪ���ص����������Դ�ĸ����������ʴ�Ϊ������
���� �����ۺϿ���ԭ��ء�����֪ʶ��Ϊ��Ƶ���㣬������ѧ���ķ��������������Ŀ��飬ע����շ�Ӧ��ԭ�����ѶȲ���

 ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�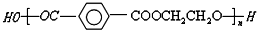 ������˵����ȷ���ǣ�������
������˵����ȷ���ǣ�������| A�� | ��ë��þ�����ά�Ļ�ѧ�ɷ���ͬ | |
| B�� | �þ�����ά����ë��һ�������¾�����ˮ�� | |
| C�� | �ϳɸþ�����ά�ĵ���Ϊ�Ա���������Ҷ��� | |
| D�� | �ɵ���ϳɸþ�����ά�ķ�Ӧ���Ӿ۷�Ӧ |
| A�� | ��aL0.1mol/L ��CH3COOH��Һ��bL0.1mol/L�� KOH��Һ��ϣ�������Һ��һ�����ڣ�c��K+��+c��H+��=c��CH3COO-��+c��OH-�� | |
| B�� | ��0.1mol/L ��NaHCO3��Һ��0.3mol/L ��Ba��OH��2��Һ�������ϣ�������Һ��һ�����ڣ�c��OH-����c��Ba+����c��Na+����c��H+�� | |
| C�� | ��1mol/L ��CH3COOH��Һ�м�������CH3COONa���壬����CH3COONaˮ���Լ��ԣ�������Һ��pH���� | |
| D�� | �����£���pH=3��CH3COOH��Һ��pH=11��NaOH��Һ�У�ˮ�ĵ���̶���ͬ |
| A�� | �����¶��ܼӿ췴Ӧ���� | |
| B�� | ����SO2��Ũ���ܼӿ췴Ӧ���� | |
| C�� | ���������O2��ʹSO2��ת���ʴﵽ100% | |
| D�� | ʹ��ǡ���Ĵ����ܼӿ췴Ӧ���� |
| A�� | ${\;}_{92}^{235}$Uԭ�Ӻ��к���92������ | |
| B�� | ${\;}_{92}^{235}$Uԭ�Ӻ�����143������ | |
| C�� | ${\;}_{92}^{235}$U��${\;}_{92}^{238}$U��Ϊͬλ�� | |
| D�� | ${\;}_{92}^{235}$Uԭ����${\;}_{92}^{238}$U��Ϊͬ�������� |
 ���׳�Ϊ�������������Ҫ�����������������㣬�ڶ������㾫����Ϊ�������Ҳ�������á���ױ�ü�ʳ�ù�ʵ�㾫�ĵ���ԭ�ϣ���������У��ѧ��ȤС����Ƶ��ɷ�����AΪ��ʼԭ���Ʊ�����������ĺϳ�·�ߣ�
���׳�Ϊ�������������Ҫ�����������������㣬�ڶ������㾫����Ϊ�������Ҳ�������á���ױ�ü�ʳ�ù�ʵ�㾫�ĵ���ԭ�ϣ���������У��ѧ��ȤС����Ƶ��ɷ�����AΪ��ʼԭ���Ʊ�����������ĺϳ�·�ߣ�

 ��
��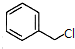 +
+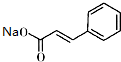 $\stackrel{��C_{2}H_{5}��_{3}N/NaI/��}{��}$
$\stackrel{��C_{2}H_{5}��_{3}N/NaI/��}{��}$ ��д�ṹ��ʽ�����ж��ֿ��ܣ�ֻдһ�֣���
��д�ṹ��ʽ�����ж��ֿ��ܣ�ֻдһ�֣���