��Ŀ����
����Ŀ���������������γɾ���ʱ��ᾧһ������ˮ���ڹ�ũҵ�����о�����Ҫ��;�����������Ӱ��������������﮵�ص�ԭ�ϵȡ�
��֪���ٲ�������������ˮ�����������Fe(SCN)63-+3C2O42-=Fe(C2O4)33-+6SCN-��
�ش��������⣺
��.��ͬѧ���ҩƷ���ָþ�����dz��ɫ����Ϊ���岻������������Ϊ���ֵ�����������Ϊ��֤�Լ��IJ��룬����ʵ����֤��ȡ�����ľ�����Ʒ����ϡ���ᣬ�μ�KSCN��Һ����Һ�����Ա仯���ɴ���Ϊ�����в�����+3�۵���������Ϊ_______�����ȷ������ȷ������������___________________________________��
��.��ͬѧΪ�ⶨ������������FeC2O4�qxH2O�еĽᾧˮ��������������װ�ã�
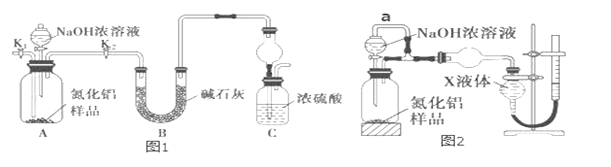
����ʵ��ǰ����Ҫ_____________________________��
�ڳ�ȡһ�������ľ��壬װ��ҩƷ����ʼʵ���������ʵ�鲽������Ϊ____________________���ظ�ʵ��ֱ��B�к��ء�
a.��ȼ�ƾ��ƣ����� b.Ϩ��ƾ��� c.�ر�K d.��K������ͨ����� e.��ȴ������ f.����
��.��ͬѧ�õζ��ķ���Ҳ���Բⶨ�������������нᾧˮ�ĺ�����ȡa�˲���������������ϡ���ᣬ�ٰ�������Һϡ�ͳ�500mL��ȡ��50mL������ƿ����������ε���δ֪Ũ�ȵ�����KMnO4��Һ����������Һ��ɫ��Ϊ�ػ�ɫ����������ð��������Һ��ɫͻ���dz��ɫ��ֹͣ�μӡ���������Һ�м����Թ�����KI��Һ�ͼ��ε�����Һ��Ȼ������c mol/L��Na2S2O3��Һ�����յ㡣����ʵ��ƽ������Na2S2O3��ҺVmL����2Na2S2O3+I2=Na2S4O6+2NaI��
��1��д������Һ�еμ�����KMnO4��Һ������Ӧ�����ӷ���ʽ_____________________
��2������������ϡ�Ͳ���������Һʱ���ձ��Ͳ������⣬������IJ���������__________
��3��x=_______________
��4����ʵ���е���KMnO4��Һ����,������xֵ___________(��ƫ��ƫС����Ӱ��)
���𰸡�����ȷ������֪C2O42-��SCN-������Fe3+��ϼ��װ��������dabecf3MnO4- + 5Fe2++5C2O42�� + 24H+ = 3Mn2+ + 5Fe3+ + 12H2O+10CO2��
�� 3MnO4- + 5Fe2++5H2C2O4 + 14H+ = 3Mn2+ + 5Fe3+ + 12H2O+10CO2��500mL����ƿ����ͷ�ιܣ�50a-72cV��/(9cV)ƫС
��������
I. ���������Ϣ��֪��C2O42-��SCN-������Fe3+���������ȡ�����ľ�����Ʒ����ϡ���ᣬ�μ�KSCN��Һ����Һ�����Ա仯�������ж������в�����+3�۵���������ȷ����ȷ��������ȷ��������֪C2O42-��SCN-������Fe3+�����
II. ����ʵ��ǰ����Ҫ���װ���Ƿ�©������ȷ�������װ����������
�� ��ȡһ�������ľ��壬װ��ҩƷ����ʼʵ���������ʵ�鲽�������ڲ����������������������ױ���������������Ҫ�ž�װ���ڵĿ�������K������ͨ�������d����Ȼ���ȼ�ƾ��ƣ�������a������Ӧ������������Ϩ��ƾ�����b������ȴ��������e�����ر�K ��c�������г�����f�����ظ�ʵ��ֱ��B�к��أ���ȷ�IJ�������Ϊ��dabecf��
III. ��1���������������к���Fe2+��C2O42���������������������ܱ�KMnO4��Һ����ΪFe3+��CO2����MnO4-����ԭΪMn2+�����ݵ����غ�������غ㼰ԭ���غ�д�����ӷ���ʽ��3MnO4- + 5Fe2++5C2O42�� + 24H+ = 3Mn2+ + 5Fe3+ + 12H2O+10CO2���� 3MnO4- + 5Fe2++5H2C2O4 + 14H+ = 3Mn2+ + 5Fe3+ + 12H2O+10CO2�� ����ȷ����3MnO4- + 5Fe2++5C2O42�� + 24H+ = 3Mn2+ + 5Fe3+ + 12H2O+10CO2���� 3MnO4- + 5Fe2++5H2C2O4 + 14H+ = 3Mn2+ + 5Fe3+ + 12H2O+10CO2�� ��
��2���Ѳ���������Һϡ�ͳ�500mL��Һʱ�����ձ��Ͳ������⣬������IJ���������500mL����ƿ����ͷ�ι�����ȷ����500mL����ƿ����ͷ�ι���
��3�����ݷ�Ӧ��3MnO4- + 5Fe2++5C2O42�� + 24H+ = 3Mn2+ + 5Fe3+ + 12H2O+10CO2����֪����Ӧ������Fe3+�ѵ���������Ϊ�ⵥ�ʣ���ӦΪ2Fe3++ 2I-= 2Fe2++ I2�����ɵĵ��ֱ�Na2S2O3��ԭΪI-����ӦΪ2Na2S2O3+I2=Na2S4O6+2NaI������������Ӧ��ϵ��֪��n(FeC2O4�qxH2O)= n(FeC2O4)= n(Fe2+)= n(Fe3+)=1/2n(I2)=n��Na2S2O3����n��Na2S2O3��= V��10-3��cmol������50mL��Һ��n(FeC2O4�qxH2O)=( V��10-3��c)mol����ԭ��ҺΪ500mL������n(FeC2O4�qxH2O)=( V��10-3��c)��(500/50)mol=10(V��10-3��c) mol��������������ag������a/(144+18x)= 10( V��10-3��c)��x=��50a-72cV��/(9cV) ����ȷ������50a-72cV��/(9cV) ��
��4����ʵ���е���KMnO4��Һ���࣬�����ӱ������������࣬���ĵ�n��Na2S2O3��= cV���࣬����x=��50a-72cV��/(9cV)��ϵ��֪��������xֵƫС����ȷ����ƫС��

 53���ò�ϵ�д�
53���ò�ϵ�д�