��Ŀ����
��11�֣���1����V L Al2(SO4)3��Һ�У���ú�Al3��������Ϊa g����Al2(SO4)3��Һ�����ʵ���Ũ��Ϊ ��SO42�������ʵ���Ũ��Ϊ ��
��2���ڱ�״���£�4 g H2��11.2 L O2��1 mL H2O�У����������������� ����ԭ���������� ������������ �������С���� ��
��3����ͬ��ͬѹ�£�ͬ����ļ��飨CH4���Ͷ�����̼������֮��Ϊ �����ʵ���֮��Ϊ ��ԭ������֮��Ϊ ������֮��Ϊ�����������������ܶ�֮��Ϊ��������������
��2���ڱ�״���£�4 g H2��11.2 L O2��1 mL H2O�У����������������� ����ԭ���������� ������������ �������С���� ��
��3����ͬ��ͬѹ�£�ͬ����ļ��飨CH4���Ͷ�����̼������֮��Ϊ �����ʵ���֮��Ϊ ��ԭ������֮��Ϊ ������֮��Ϊ�����������������ܶ�֮��Ϊ��������������
��1��a/54Vmol/L��a/18Vmol/L����2��H2��H2��O2��H2O��
��3��1��1 1��1 5��3 4��11 4��11
��3��1��1 1��1 5��3 4��11 4��11
����һ�����ʵ���Ũ����Һ�����ơ�
��1��Al3��������Ϊa g���������ӵ����ʵ�����a/27mol�����Ը��ݻ�ѧʽ��֪��Al2(SO4)3�����ʵ�����a/54mol������������Ũ����a/54Vmol/L�������������Ļ�ѧʽ��֪��SO42�������ʵ���Ũ��Ϊa/54Vmol/L��3/2��a/18Vmol/L��
��2���ڱ�״���£�4 g H2��11.2 L O2��1 mL H2O�����ʵ����ֱ���2mol��0.5mol��1/18mol������������������������������ԭ��������������������4mol��ԭ�ӣ�������ˮ�������ֱ���16g��1g�����������������������������С����ˮ����3��
��3�����ݰ����ӵ����ɿ�֪����ͬ��ͬѹ�£�ͬ����ļ��飨CH4���Ͷ�����̼������֮��Ϊ1�U1�����ʵ���֮��Ҳ��1�U1��ԭ������֮��Ϊ1��5�U1��3��5�U3������֮��Ϊ1��16�U1��44��4�U11������ͬ�����£�������ܶ�֮����Ħ������֮�ȣ����Զ��ߵ��ܶ�֮����16��44��4�U11��
��1��Al3��������Ϊa g���������ӵ����ʵ�����a/27mol�����Ը��ݻ�ѧʽ��֪��Al2(SO4)3�����ʵ�����a/54mol������������Ũ����a/54Vmol/L�������������Ļ�ѧʽ��֪��SO42�������ʵ���Ũ��Ϊa/54Vmol/L��3/2��a/18Vmol/L��
��2���ڱ�״���£�4 g H2��11.2 L O2��1 mL H2O�����ʵ����ֱ���2mol��0.5mol��1/18mol������������������������������ԭ��������������������4mol��ԭ�ӣ�������ˮ�������ֱ���16g��1g�����������������������������С����ˮ����3��
��3�����ݰ����ӵ����ɿ�֪����ͬ��ͬѹ�£�ͬ����ļ��飨CH4���Ͷ�����̼������֮��Ϊ1�U1�����ʵ���֮��Ҳ��1�U1��ԭ������֮��Ϊ1��5�U1��3��5�U3������֮��Ϊ1��16�U1��44��4�U11������ͬ�����£�������ܶ�֮����Ħ������֮�ȣ����Զ��ߵ��ܶ�֮����16��44��4�U11��

��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
�����Ŀ
 MnCl2+C12��+2H2O
MnCl2+C12��+2H2O
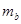 �����<����>������=������
�����<����>������=������