��Ŀ����
ij�о���ѧϰС���ͬѧ̽����ҵ����������ͭ�Ͻ���ϵ������ã���ͬѧ��Ƶ�ʵ�鷽�����£�
��ش�
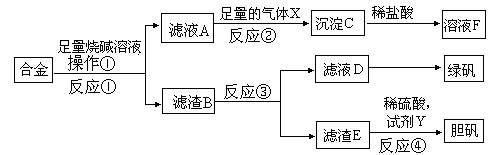
��1��д����Ӧ�ڵ����ӷ�Ӧ����ʽ__________________________________________��
��2��������E�м���ϡ������Լ�Y�Ƶ���������һ����ɫ��ѧ���գ��Լ�YΪ��ɫҺ�壬��Ӧ�ܵ��ܻ�ѧ����ʽ��_____________________________________��
��3����ͬѧ�ڼ�ͬѧ�����Ļ��������������B���Ʊ� FeCl3??6H2O���壬�ǽ������Ȼ�����Һ�ü���Ũ�������½ᾧ���Ƶ�FeCl3??6H2O���壬������ֱ�������ᾧ�ķ������Ƶþ����������__________________����������������������������������
��4����ͬѧ������·����ⶨ��ͬѧ���Ƶõ��̷������нᾧˮ�������䷽����������
�ٳ�ȡmg����ϸ�����Ƶõ��̷����壬�����ķ�����___________
a��ֱ�ӽ������������ƽ�����������ֽƬ�ϳ���
b�����������ڸ���������У�Ȼ���ٰ�a�в������г���
c�����������ڼ�ȷ�����ĸ��������У�Ȼ���ٰ�a�в������г���
���ڵ����������м��������о���ʹ��ʧȥȫ���ᾧˮ����ֹͣ���ȣ������ڵ�������������ȴ�����ڵ������м�������ȴ��ԭ����____________________________
�۽�����������ƽ�Ͻ��г������Ƶ�����Ϊn1g
���ظ��ڡ��۵IJ������Ƶõ�����Ϊn2g����������Ҫ�ټ����ٳ�����������________
���±��������Ǹ�ͬѧʵ�鱨���е����ݣ������������X=_______
| ʵ���� | �������� | �����뾧�������� | ���Ⱥ���������������� |
| 1 | 11��2g | 25��1g | 19��2g |
| 2 | 11��2g | 25��1g | 18��8g |
| 3 | 11��2g | 25��1g | 18��9g |
��1��AlO2- + CO2 + 2H2O= Al��OH��3��+ HCO3-
��2��Cu + H2O2 + H2SO4 + 3H2O = CuSO4��5H2O��Cu+H2O2 + H2SO4 = CuSO4 + 2H2O
��3����Ϊ�Ȼ�����ǿ��������ˮ�������������������ᣬ��������ʱʹHCl �ӷ����ˮ����е��ף���˵ò���FeCl3??6H2O����
��4����c ��2�� �ڷ�ֹ������Fe2������������n1��n2��0��1g�� ��7
����:
���ֽ�����ֻ��������Ӧ����NaAlO2��ͨ������CO2������Al(OH)3��Һ��NaHCO3����֪E��ͭ��ͭ��ϡ�����Ӧ����Y�н�ǿ�������ԣ������ΪҺ��֪ΪH2O2��D��FeSO4������NaOH ������Fe2����FeCl3�ǻӷ�����������Σ�ֱ�Ӽ�������Һʱ�ܴ�ʹˮ����е��ס�
Ӧ�ȳ��������������ٳ�������ҩƷ�����ͣ����������¶Ƚϸߵ��������ױ�������������Ӧ���ڵ������н���ʵ�飻���ٴγ����������n1������0��1gʱ��������Ҫ����2��3���γ�������������һ���������������ϴ���2��3���γ��������ƽ��ֵ���м��㡣

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���1��д��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ��
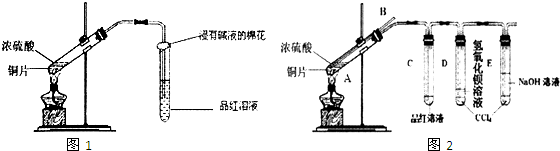
��2����С��ͬѧ��ʵ���з�������ʵ��װ�����൱���֮������ʵ�鲻����ȫ������ɻ�����Ⱦ�ȣ�Ϊ�Ľ�ʵ�������˽�SO2�����ʣ�����ͬѧ�ʵ����ۺ�����ʦ�Ľ������������ͼ2ʵ��װ�ã�
�����������B������
�ڶ��Թ�A�е�ŨH2SO4��ͭƬ���м��ȣ�����E�Թ����������ݳ���Ʒ����Һ�ܿ���ɫ��������δ��D�Թ�������������Һ���ֻ��ǣ�Ϊ̽��D�Թ���δ���ֻ��ǵ�ԭ��С��ͬѧ�ڻ�ѧ�ֲ���ֻ���ĵ��������ʳ����µ��ܽ�����ݣ�
| ���� | �ܽ�ȣ�g/100ˮ�� | ���� | �ܽ�ȣ�g/100ˮ�� |
| Ca��OH��2 | 0.173 | Ba��OH��2 | 3.89 |
| CaCO3 | 0.0013 | BaSO3 | 0.016 |
| Ca��HCO3��2 | 16.60 |
��Ϊ��֤D�Թ�����Һ����ɣ�����������ʵ�飬����������������ʵ�����ݣ�
| ʵ�鷽�� | ���� |
| 1��ȡ������Һ���Թ��У�����ϡ���ᣬ���ȣ� ��ʪ�����ɫʯ����ֽ�������ɵ����壮 |
|
| 2��ȡ������Һ���Թ��У����� |
 ������Ƥ�����н�ǿ����ʴ�ԣ��������г��õĽ�������֮һ����п��������Ƥ�ı����㣬���Ĥ�ĺ�ȼ����ȶ�Ҳ�����ж϶Ʋ���������Ҫָ�꣮ij�о���ѧϰС��Ϊ�˲ⶨ��п��Ƥ�ĺ�ȣ�����������ʵ�鷽����
������Ƥ�����н�ǿ����ʴ�ԣ��������г��õĽ�������֮һ����п��������Ƥ�ı����㣬���Ĥ�ĺ�ȼ����ȶ�Ҳ�����ж϶Ʋ���������Ҫָ�꣮ij�о���ѧϰС��Ϊ�˲ⶨ��п��Ƥ�ĺ�ȣ�����������ʵ�鷽���� Al��OH��3+3H+��Cu2++2H2O
Al��OH��3+3H+��Cu2++2H2O Cu��OH��2+2H+��2Al+6H+=2Al3++3H2��
Cu��OH��2+2H+��2Al+6H+=2Al3++3H2��