��Ŀ����
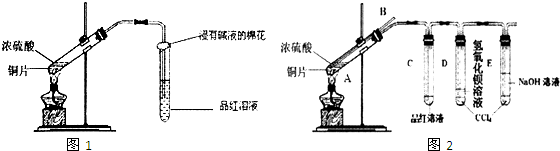
ij�о���ѧϰС��Ϊ̽��Cu��ŨH2SO4�ķ�Ӧ�������SO2�����ʣ������ͼ1ʵ��װ�ã���1��д��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ��
��2����С��ͬѧ��ʵ���з�������ʵ��װ�����൱���֮������ʵ�鲻����ȫ������ɻ�����Ⱦ�ȣ�Ϊ�Ľ�ʵ�������˽�SO2�����ʣ�����ͬѧ�ʵ����ۺ�����ʦ�Ľ������������ͼ2ʵ��װ�ã�
�����������B������
�ڶ��Թ�A�е�ŨH2SO4��ͭƬ���м��ȣ�����E�Թ����������ݳ���Ʒ����Һ�ܿ���ɫ��������δ��D�Թ�������������Һ���ֻ��ǣ�Ϊ̽��D�Թ���δ���ֻ��ǵ�ԭ��С��ͬѧ�ڻ�ѧ�ֲ���ֻ���ĵ��������ʳ����µ��ܽ�����ݣ�
| ���� | �ܽ�ȣ�g/100ˮ�� | ���� | �ܽ�ȣ�g/100ˮ�� |
| Ca��OH��2 | 0.173 | Ba��OH��2 | 3.89 |
| CaCO3 | 0.0013 | BaSO3 | 0.016 |
| Ca��HCO3��2 | 16.60 |
��Ϊ��֤D�Թ�����Һ����ɣ�����������ʵ�飬����������������ʵ�����ݣ�
| ʵ�鷽�� | ���� |
| 1��ȡ������Һ���Թ��У�����ϡ���ᣬ���ȣ� ��ʪ�����ɫʯ����ֽ�������ɵ����壮 |
|
| 2��ȡ������Һ���Թ��У����� |
��������1������Ũ�����ǿ�����Լ���ԭ�����������д����Ӧʽ�����������Ϊ����������ж������ü�Һ���շ�ֹ��Ⱦ������
��2���ٿ��Դ�ʵ�鰲ȫ�Ƕȷ���װ���г����ܵ����ã�
�ڸ��ݱ���CaCO3��Ca��HCO3��2���ܽ�����Ba��HSO3��2���ܽ�ȣ��ɴ˽�������
�۸���Ba��HSO3��2���������ơ����ᷴӦԭ�����ƲⷴӦ����
��2���ٿ��Դ�ʵ�鰲ȫ�Ƕȷ���װ���г����ܵ����ã�
�ڸ��ݱ���CaCO3��Ca��HCO3��2���ܽ�����Ba��HSO3��2���ܽ�ȣ��ɴ˽�������
�۸���Ba��HSO3��2���������ơ����ᷴӦԭ�����ƲⷴӦ����
����⣺��1��Ũ�����ͭ����������ͭ����������ԭ�ɶ�������Ӧ����ʽΪ��Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O�����������ж������������������ͨ�ԣ�����Ӧ�����κ�ˮ�����Կ��ü�Һ���ն�������ֹ��Ⱦ������
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O������SO2��ֹ��Ⱦ������
��2���ٴӰ�ȫ�Ƕȿ��ǿ��ܷ��ӵ����ã��ӷ�������������������ƽ������ѹǿ�����ã��ӻ����Ƕȿ���װ������δ�ų��Ķ������ɴ�B����ͨ��������Ա��װ���в����Ķ��������ų��������գ���ֹ���װ��ʹ����������Ⱦ������
�ʴ�Ϊ����ֹC�е�Һ�嵹��������鷴Ӧʱ�����Ƿ�������жװ��ǰ��B�ܿ����Թ�A�д������������ž�A�е�SO2���壬���������Ⱦ�ȣ���
�ڸ��ݱ���CaCO3��Ca��HCO3��2���ܽ�ȿ�֪��Ca��HCO3��2���ܽ��Զ����CaCO3���ɴ����Ba��HSO3��2���ܽ��Զ����BaSO3��Ԥ����D�Թ�δ���ֻ��ǵ�ԭ�����������ܽ�Ƚϴ��Ba��HSO3��2��
�ʴ�Ϊ���������ܽ�Ƚϴ��Ba��HSO3��2��
��Ba��HSO3��2��HCl��Ӧ�ķ���ʽΪ��Ba��HSO3��2+2HCl�TBaCl2+2H2O+2SO2������������Ϊ����������ʯ���죬��������Ϊ���Թ��������ݲ�����ʪ�����ɫʯ����ֽ��죻
Ba��HSO3��2Ϊ��ʽ�Σ��ܹ�������������Һ��Ӧ���������ᱵ���������Լ����Լ�Ϊ����������Һ����Ӧ�ķ���ʽΪ��Ba��HSO3��2+2NaOH�TBaSO3��+H2O+Na2SO3�����Կ���������Ϊ�л��dz��֣�
�ʴ�Ϊ���Թ��������ݲ�����ʪ�����ɫʯ����ֽ��죻NaOH��Һ���Թ��г��ֻ��ǣ�
| ||
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
| ||
��2���ٴӰ�ȫ�Ƕȿ��ǿ��ܷ��ӵ����ã��ӷ�������������������ƽ������ѹǿ�����ã��ӻ����Ƕȿ���װ������δ�ų��Ķ������ɴ�B����ͨ��������Ա��װ���в����Ķ��������ų��������գ���ֹ���װ��ʹ����������Ⱦ������
�ʴ�Ϊ����ֹC�е�Һ�嵹��������鷴Ӧʱ�����Ƿ�������жװ��ǰ��B�ܿ����Թ�A�д������������ž�A�е�SO2���壬���������Ⱦ�ȣ���
�ڸ��ݱ���CaCO3��Ca��HCO3��2���ܽ�ȿ�֪��Ca��HCO3��2���ܽ��Զ����CaCO3���ɴ����Ba��HSO3��2���ܽ��Զ����BaSO3��Ԥ����D�Թ�δ���ֻ��ǵ�ԭ�����������ܽ�Ƚϴ��Ba��HSO3��2��
�ʴ�Ϊ���������ܽ�Ƚϴ��Ba��HSO3��2��
��Ba��HSO3��2��HCl��Ӧ�ķ���ʽΪ��Ba��HSO3��2+2HCl�TBaCl2+2H2O+2SO2������������Ϊ����������ʯ���죬��������Ϊ���Թ��������ݲ�����ʪ�����ɫʯ����ֽ��죻
Ba��HSO3��2Ϊ��ʽ�Σ��ܹ�������������Һ��Ӧ���������ᱵ���������Լ����Լ�Ϊ����������Һ����Ӧ�ķ���ʽΪ��Ba��HSO3��2+2NaOH�TBaSO3��+H2O+Na2SO3�����Կ���������Ϊ�л��dz��֣�
�ʴ�Ϊ���Թ��������ݲ�����ʪ�����ɫʯ����ֽ��죻NaOH��Һ���Թ��г��ֻ��ǣ�
���������⿼�������ἰ������������ʣ���Ŀ�Ѷ��еȣ�ע������Ũ����Ļ�ѧ���ʡ���������ļ��鷽���������ʱע��������ͼʾ��ͼ����Ϣ�������Ŀ���ʽ��з�����

��ϰ��ϵ�д�
 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
�����Ŀ
