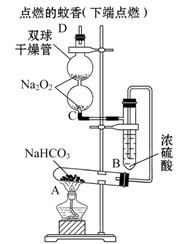
��Ŀ����
ij��ѧС��������װ�ö������仯��������ʽ���̽�����ش��й����⣺
��1����С��ͬѧ���Ƶ�����������������ϳ�ʱ�䣬����ͼ��a����ʾװ�ö�����в�����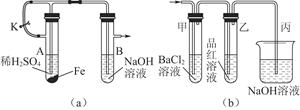
��ijͬѧ�IJ����ǣ��ȼн�ֹˮ��K����ʹA�ܿ�ʼ��Ӧ��ʵ������B���й۲쵽��������
��
��Ϊ�ﵽʵ��Ŀ�ģ���ȷ�IJ����� ��
B���з�����Ӧ�����ӷ���ʽ�� ��
��2��������װ�õ�ʵ�������ȡA���ڷ�Ӧ���õ���Һ����������С�����ɺ�õ�FeSO4���ٸ������գ��й�װ�úͲ���������ȥ����������º���ɫ���壬���ֽ�ʱ���������尴ͼ��b����ʾװ������ͨ��ϴ��װ�ã�����Թܼ��ڳ��ְ�ɫ�������Թ�����Ʒ����Һ��ɫ��ȥ���ش�
���÷���ʽ��ʾ�Թܼײ�����ɫ������ԭ��
��
��Ϊ��֤����ɫ����ɷ֣��ɽ������²���
��
��ͼ��b���б�װ�õ������� ��
�ܸ���ʵ������д��A������Һ���ɺ��ڸ������շֽ�ʱ��������Ӧ�Ļ�ѧ����ʽ ��
��1����A����Һ����B�У���ʼ������ɫ������Ȼ���Ϊ����ɫ������ɺ��ɫ
�ڴ�ֹˮ��a��ʹA�ܷ�Ӧһ��ʱ����B�е����о������ݲ������ټн�ֹˮ��a
Fe2����2OH��=Fe��OH��2�� ���������кͷ�Ӧд��д���ɣ�
��2����SO3��H2O=H2SO4��
H2SO4��BaCl2=BaSO4����2HCl
��SO3��H2O��BaCl2=BaSO4����2HCl
��ȡ��������������ٵμ�KSCN��Һ���۲쵽��Һ���ɫ
������SO2
��2FeSO4 Fe2O3��SO3����SO2��
Fe2O3��SO3����SO2��
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д��ú���Al2O3��SiO2������FeO��xFe2O3�������Ʊ�Al2(SO4)3��18H2O��������������(���ֲ�����������)��
��.�������м������ϡH2SO4�����ˣ�
��.����Һ�м������KMnO4��Һ��������Һ��pHԼΪ3��
��.���ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ��
��.����MnSO4���Ϻ�ɫ��ʧ�����ˣ�
��.Ũ�����ᾧ�����룬�õ���Ʒ��
��1��H2SO4�ܽ�Al2O3�����ӷ���ʽ��____________________________________��
��2����MnO4-����Fe2�������ӷ���ʽ����������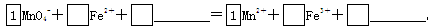
��3����֪��
�����������������pH
| | Al(OH)3 | Fe(OH)2 | Fe(OH)3 |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
���ݱ������ݽ��Ͳ�����Ŀ�ģ�________��
��4����֪��һ�������£�MnO4-����Mn2����Ӧ����MnO2��
�� �� �� �ij����м���ŨHCl�����ȣ���˵�������д���MnO2��������__________��
�ڢ� �м���MnSO4��Ŀ����_____________________________________________��
Fe2����I�������ֳ����Ļ�ԭ�����ӡ�
��1����FeSO4��Һ�еμ���ˮ����Һ��dz��ɫ��ɻ�ɫ����Ӧ�����ӷ���ʽΪ ����KI��Һ�еμ���ˮ����Һ����ɫ��ɻ�ɫ����Ӧ�����ӷ���ʽ�� ��
��2������FeSO4��Һ��KI��Һ����ˮΪ�Լ���֤I���Ļ�ԭ��ǿ��Fe2�������ʵ�鷽�����������ʵ�鲽�衢Ԥ������ͽ��ۡ�������ѡ�Լ���3 mol��L��1 H2SO4��0.01 mol��L��1 KMnO4��20% KSCN��3%H2O2��������Һ����ɫʯ����Һ��
| ʵ�鲽�� | Ԥ����������� |
| ����1��ȡ2mLFeSO4��Һ��2mLKI��Һ������Թ��У��ٵμ�1��2����ˮ�� | �� |
| ����2��____________________________________ ____________________________________�� | |
��3�����ã�2���ṩ���Լ�֤���������Ļ����������ԣ�2�ۣ�ʵ������������ǣ�ȡ������Ʒ����ˮ�� ��
����������FeC2O4�������������Լ�����Ӱ���Լ����͵�ز�����������﮵����������������ڸ�������ʱ�����ܹ��ֽ�,��ȤС��Բ��������ķֽ���������ʵ���̽��������֪��CO�����Ȼ���[PdC12]��Һ��Ӧ���ɺ�ɫ���ٷۡ���
��1�������������ֽ�������������ͨ������ʯ��ˮ���Ȼ�����Һ���۲쵽����ʯ��ˮ����ǣ��Ȼ�����Һ���к�ɫ�������ɡ�˵������������� �����ѧʽ��
��2��̽�����������ֽ�õ��ĺ�ɫ�����������Ԫ�صĴ�����ʽ��
���������⡿
���������ֽ��õ��ĺ�ɫ������ʲô��
��������衿
����1�� ������2��FeO������3��FeO��Fe�Ļ���
��ʵ�鷽����
��ѡ�Լ������ᡢ��ˮ��CuSO4��Һ��KSCN��Һ������ˮ��
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ����1�����Թ��м��������������ټ������� ������� | ����Һ��ɫ���Ըı䣬 ���к�ɫ�������ɡ� | ��Fe���ڡ� |
| ����2��������1�еõ�����Һ���ˣ���������ˮ������ϴ����ϴ��Һ����ɫ�� | | |
| ����3��ȡ����2�õ��������������Թ��У��μӹ������ᣬ���ú�ȡ�ϲ���Һ�� �� | �� | ��FeO���ڡ� |
����˼������
����ȤС�����۷�����Ϊ����������ֱ�ӷֽ����ù������Ӧ����FeO�������չ�������л�����Fe����Ϊ ��д��ѧ����ʽ����
��3������ʵ��̽���ͷ�˼��д�����������ڸ�������ʱ����ֱ�ӷֽ�Ļ�ѧ����ʽ ��
ijУ��ѧ��ȤС��Ϊ̽������Ũ����ķ�Ӧ,�����ͼ1��ͼ2��ʾװ�ý���ʵ�顣 
��1����˵����SO2���������ʵ�������� ��
��2��ͼ2�е�����e����Ҫ����Ϊ ��
��3������װ����ͼ2�е�NaOH��Һ������SO2β������ֹ��Ⱦ���罫�����Ϊ����KMnO4��Һ��ͬ�����ԴﵽĿ�ģ���д������KMnO4��Һ��SO2��Ӧ�Ļ�ѧ����ʽ��
��
��4���Ա�����ʵ��װ�ã����ѷ���ͼ2װ�ó����ܸ��õ������ж�����SO2��ֹ����Ⱦ�����⣬����һ���dz����Ե��ŵ㣬����Ϊ�� ��
��5����Ӧһ��ʱ���ֹͣ��Ӧ������ȴ���ý�ͷ�ι���ȡA�Թ��е���Һ���뵽����ˮ����Ϊ�����������������������ӵijɷ����������ֿ��ܣ�
��ֻ����Fe3+����ֻ����Fe2+�� ����Fe3+����Fe2+��
Ϊȷ����Һ�ijɷ֣�ѡ�������Լ���
| A��ϡHCl��Һ | B��ϡ���� | C��KSCN��Һ | D������KMnO4��Һ |
�����������ص�ʵ��̽����
| ʵ�鲽�� | ʵ�������� |
| 1��ȡһ֧�ྻ���Թܣ��μ�1-2mL��������Һ�������Թ��еμӼ���KSCN��Һ | ��1�� ����˵��������� ��2�� ����˵����Һ�д���Fe3+������������ |
| 2�� | �� |