��Ŀ����
����Ŀ��������ء��߸�����һ�ֵ��͵�ǿ�����������������գ�
��1��ijһ��Ӧ��ϵ�з�Ӧ��������ﹲ�������ʣ�O2��H2CrO4��Cr(OH)3��H2O��H2O2 ��֪�÷�Ӧ��H2O2ֻ�������¹��̣�H2O2��O2
�ٸ÷�Ӧ�еĻ�ԭ����_____________��
�ڸ÷�Ӧ�У�������ԭ��Ӧ�Ĺ�����____________��__________��
��д���÷�Ӧ�Ļ�ѧ����ʽ_______________________��
��2������KMnO4������Һ����Cu2S��CuS�Ļ����ʱ�������ķ�Ӧ���£�
��MnO4-��Cu2S��H����Cu2����SO2����Mn2����H2O(δ��ƽ)
��MnO4-��CuS��H����Cu2����SO2����Mn2����H2O(δ��ƽ)
���й��ڷ�Ӧ�ٵ�˵������ȷ����______________(����ĸ���)��
a����������Ԫ����Cu��S
b���������뻹ԭ�������ʵ���֮��Ϊ8��5
c������2.24 L(�����)SO2����Ӧת�Ƶ��ӵ����ʵ�����0.8 mol
d����ԭ�Ե�ǿ����ϵ�ǣ�Mn2����Cu2S
��3����ϡ�����У�![]() ��H2O2Ҳ�ܷ���������ԭ��Ӧ��
��H2O2Ҳ�ܷ���������ԭ��Ӧ��
��֪��2KMnO4��7H2O2��3H2SO4===K2SO4��2MnSO4��6O2����10H2O����2 mol KMnO4������H2O2��____________mol��
��4�����������Һ�����������������·�Ӧ��
10FeS��6KMnO4��24H2SO4===3K2SO4��6MnSO4��5Fe2(SO4)3��10S��24H2O
��������Ӧǰ����������������2.8 g������Ԫ����KMnO4֮�䷢������ת�Ƶ���ĿΪ________��
���𰸡�H2O2 H2CrO4 Cr(OH)3 3H2O2 +2H2CrO4= 2Cr(OH)3+ +3O2��+2H2O a b c 5 0.1NA(��6.02��1022)
��������
��1��H2O2ֻ�������¹��̣�H2O2��O2��OԪ�صĻ��ϼ����ߣ���CrԪ�صĻ��ϼ۽��ͣ���ԭ����ΪH2CrO4��Cr��OH��3������H2CrO4+H2O2��Cr��OH��3+H2O+O2������ϵ����غ㼰�����غ㶨�ɷ�����
��2����MnO4��+Cu2S+H����Cu2��+SO2��+Mn2��+H2O�У�MnԪ�صĻ��ϼ۽��ͣ���ͭԪ�صĻ��ϼ���+1����+2�ۣ���Ԫ�صĻ��ϼ���-2����+4�ۣ����������ԭ��Ӧ���������ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ļ�ԭ�Է�����
��3������������ԭ��Ӧ���������ͻ�ԭ��֮���ʧ���ӵ����ʵ�����ȼ��㣻
��4����Ӧ10FeS+6KMnO4+24H2SO4��3K2SO4+6MnSO4+5Fe2��SO4��3+10S+24H2O�У���Ӧǰ�����仯Ϊ��FeS��S��������ٵ�����ʵ��������Ԫ�ص��������ݴ˼�����������ʵ�����ת�Ƶĵ�������
��1������OԪ�صĻ��ϼ����߿�֪��H2O2Ϊ��ԭ����
�ڸ÷�Ӧ�У�CrԪ�صĻ��ϼ۽��ͣ���ԭ����ΪH2CrO4��Cr��OH��3��
�۷���H2CrO4+H2O2��Cr��OH��3+H2O+O2����CrԪ�صĻ��ϼ���+6�۽���Ϊ+3�ۣ�OԪ�صĻ��ϼ���-1������Ϊ0��
�ɵ����غ��֪2H2CrO4+3H2O2��2Cr��OH��3+H2O+3O2����
����Hԭ���غ��֪��3H2O2+2H2CrO4=2Cr��OH��3+3O2��+2H2O
����������е�������Ӧ�������÷�Ӧ�Ļ�ѧ����ʽ3H2O2 +2H2CrO4= 2Cr(OH)3+ +3O2��+2H2O��
��2��a��ͭԪ�صĻ��ϼ���+1����+2�ۣ���Ԫ�صĻ��ϼ���-2����+4�ۣ�������������a��ȷ��
b���������������ʵ���Ϊx����ԭ�������ʵ���Ϊy����5x=��2+6��y��x:y=8:5����b��ȷ��
c������b�������5 mol SO2ʱ��ת�Ƶ���40 mol�����Ե���0.1 mol SO2����ʱ��ת�Ƶ���0.8 mol����c��ȷ��
d�����ݷ���ʽ����ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ļ�ԭ�ԣ���ԭ��Cu2S��Mn2������d����
�ʴ�Ϊ��abc��
��3����Ӧ2KMnO4+7H2O2+3H2SO4��K2SO4+2MnSO4+6O2��+10H2O�У�2molKMnO4������5molH2O2�����ɵ�6molO2�У���5molΪH2O2���������ɣ���1molΪH2O2��������������ԭ��Ӧ���ɣ���2 mol KMnO4������H2O2��5mol��
��4��10FeS��6KMnO4��24H2SO4===3K2SO4��6MnSO4��5Fe2(SO4)3��10S��24H2O
��Ӧ�й��������仯Ϊ��FeS��S�����������仯Ϊ��Ԫ�صı仯����Ӧǰ����������������2.8g���μӷ�Ӧ���������ӵ����ʵ���Ϊ��2.8g��56g��mol��1=0.05mol������������FeS�����ʵ���Ϊ0.05mol�����������������������Ԫ��Ԫ�ص����ʵ���Ϊ0.05mol��ת�Ƶĵ��ӵ����ʵ���Ϊ��0.05mol��2=0.1mol��������ĿΪ��0.1NA��

 �Ķ��쳵ϵ�д�
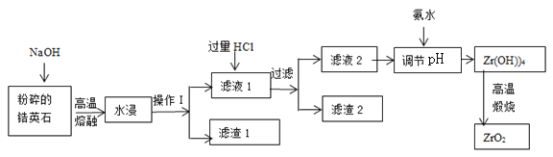
�Ķ��쳵ϵ�д�����Ŀ�������(ZrO2)���Ͼ��и�Ӳ�ȡ����۵㡢��ǿ�ȡ������ԡ����ߵ���ĥ�Լ��ͻ�ѧ��ʴ�Ե��������ﻯ���ܡ����Ӣʯ(��Ҫ�ɷ�Ϊ ZrSiO4����������Al2O3��SiO2��Fe2O3������)Ϊԭ��ͨ�����۷��Ʊ������(ZrO2)���������£�
25��ʱ���й�������ˮ��Һ�г���ʱ��pH���ݣ�
Fe(OH)3 | Zr(OH)4 | Al(OH)3 | |
��ʼ����ʱpH | 1.9 | 2.2 | 3.4 |
������ȫʱpH | 3.2 | 3.2 | 4.7 |
��ش��������⣺
(1)������ּ�����ѧ��Ӧ���ʵĴ�ʩ��________________________________��
(2)����I��������__________________������1�ɷ�Ϊ_________������2�ijɷ�Ϊ_____________��
(3)�Ӣʯ��������������ת��ΪNa2ZrO3��д���÷�Ӧ�Ļ�ѧ����ʽ��____________________��
(4)������pH��ʱ�����ʵ�pH��Χ��__________________��Ϊ�˵õ�����ZrO2��Zr(OH)4��Ҫϴ�ӣ�����Zr(OH)4�Ƿ�ϴ�Ӹɾ��ķ�����__________________��
(5)д�����������������̵Ļ�ѧ����ʽ________________________________������ZrO2�����ʣ��Ʋ���һ����;________________________________��
����Ŀ�����и��������У��������ͼʾ������һ��������һ��ת����ϵ������У� ��
��� | X | Y | Z | W |
|
�� | Si | Na2SiO3 | H2SiO3 | SiO2 | |
�� | Na | NaOH | Na2CO3 | NaCl | |
�� | Cl2 | Ca(ClO)2 | HClO | HCl | |
�� | Fe | FeCl3 | FeCl2 | Fe(OH)2 |
A.�ڢ�B.�٢ۢ�C.�٢�D.�٢ڢ�