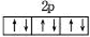��Ŀ����
�ڣ�Te��Ϊ��A��Ԫ�أ��ǵ�����¼����²��ϵ���Ҫ�ɷ�֮һ����ҵ�Ͽɴӵ�⾫��ͭ������������ȡ�ڡ�
��1����ͭ�к���Cu������Zn��Ag��Au��TeO2�������������⾫��������������Ҫ����TeO2�������������ʼ������������⾫����ͭʱ�������缫��ӦʽΪ ��
��2��TeO2���������������ˮ��������ǿ���ǿ�����������������ȡ�ڵ�һ�ֹ����������£�
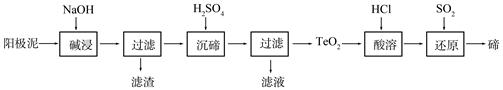
�١������ʱTeO2������Ӧ�Ļ�ѧ����ʽΪ ��
�ڡ����ڡ�ʱ������Һ��pHΪ4.5-5.0������TeO2���������H2SO4��������Һ��ȹ��������ڵij�������ȫ��ԭ���� ����ֹ�ֲ���ȹ���IJ��������� ��
�ۡ����ܡ���SO2ͨ��TeCl4��Һ�н��С���ԭ���õ��ڣ��÷�Ӧ�Ļ�ѧ����ʽ�� ��
��1����ͭ�к���Cu������Zn��Ag��Au��TeO2�������������⾫��������������Ҫ����TeO2�������������ʼ������������⾫����ͭʱ�������缫��ӦʽΪ ��
��2��TeO2���������������ˮ��������ǿ���ǿ�����������������ȡ�ڵ�һ�ֹ����������£�

�١������ʱTeO2������Ӧ�Ļ�ѧ����ʽΪ ��
�ڡ����ڡ�ʱ������Һ��pHΪ4.5-5.0������TeO2���������H2SO4��������Һ��ȹ��������ڵij�������ȫ��ԭ���� ����ֹ�ֲ���ȹ���IJ��������� ��
�ۡ����ܡ���SO2ͨ��TeCl4��Һ�н��С���ԭ���õ��ڣ��÷�Ӧ�Ļ�ѧ����ʽ�� ��
��1��Zn-2e-=Zn2+ Cu-2e-=Cu2+ ����4�֣���2�֣�
��2���� TeO2+2NaOH=Na2TeO3+H2O ��3�֣�
��TeO2�����������H2SO4�����ᵼ��TeO2������H2SO4��Ӧ������ʧ����3�֣�
��������H2SO4�������Ͻ��� ��3�֣�
�� TeCl4 + 2SO2 + 4H2O="Te" + 4HCl + 2H2SO4 ��3�֣�
��2���� TeO2+2NaOH=Na2TeO3+H2O ��3�֣�
��TeO2�����������H2SO4�����ᵼ��TeO2������H2SO4��Ӧ������ʧ����3�֣�
��������H2SO4�������Ͻ��� ��3�֣�
�� TeCl4 + 2SO2 + 4H2O="Te" + 4HCl + 2H2SO4 ��3�֣�
�����������1����⾫����ͭʱ����ͭ�е�Cu������Zn����������������Ӧ��Zn��Cu���ã���ʧ���ӣ����������缫��ӦʽΪZn-2e-=Zn2+ Cu-2e-=Cu2+
��2����TeO2��������������������Ʒ����������������������Ƶķ�Ӧ����ѧ����ʽΪTeO2+2NaOH=Na2TeO3+H2O��
����ΪTeO2�����������H2SO4�����ᵼ��TeO2������H2SO4��Ӧ������ʧ����ֹ�ֲ���ȹ���IJ��������ǻ�������H2SO4�������Ͻ��裻
��SO2��ԭTeCl4ΪTe������������Ϊ���ᣬ��ѧ����ʽΪTeCl4+2SO2+4H2O=Te+4HCl+2H2SO4

��ϰ��ϵ�д�
 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�
�����Ŀ
 (RE)2(SO4)3��Na2SO4��xH2O����������������ȷ���ǣ� ��
(RE)2(SO4)3��Na2SO4��xH2O����������������ȷ���ǣ� ��
 ͨ������
ͨ������ ��Һ�У���Һ����ɫ������ɫ����Ӧ��������Ԫ�ش�����ʽ�������� ��
��Һ�У���Һ����ɫ������ɫ����Ӧ��������Ԫ�ش�����ʽ�������� ��