��Ŀ����
��Ӧ�����Ժ��������ȡ�����Ҫ��Ӧ����Ӧ������������ʯ����ȡ�����Ҫ��Ӧ����2NaI��MnO2��3H2SO4=2NaHSO4��MnSO4��2H2O��I2����2NaIO3��5NaHSO3=2Na2SO4��3NaHSO4��H2O��I2����֪NaIO3����������MnO2����������й�˵����ȷ���� (����)��
| A��I2�ڷ�Ӧ�����ǻ�ԭ����ڷ�Ӧ�������������� |
| B��������Ӧ�����ɵ�����I2ʱת�Ƶĵ�������� |
| C��NaI��NaIO3��һ���������ܷ�Ӧ����I2 |
| D��NaIO3�ڻ�ѧ��Ӧ��ֻ������������������ԭ�� |
C
��Ӧ����������ΪMnO2����ԭ��ΪNaI��I2���������MnSO4�ǻ�ԭ�����Ӧ����I2�ǻ�ԭ���NaHSO4���������A���������1 mol I2ʱ����Ӧ��ת�Ƶ���2 mol����Ӧ��ת�Ƶ���10 mol��B�������ΪNaIO3����������MnO2��������Դӷ�Ӧ�ٿ��Է����ƶ�C����ȷ��NaIO3�е�Ԫ�ز�����ۣ���������������Ҳ��������ԭ����D�����

��ϰ��ϵ�д�
�����Ŀ
 2H++CO32-
2H++CO32-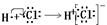
 2CrO42��(��ɫ) +2H+
2CrO42��(��ɫ) +2H+ HCOONa+H2O�������й�˵������ȷ����( )
HCOONa+H2O�������й�˵������ȷ����( ) 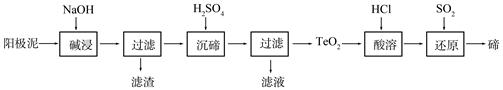
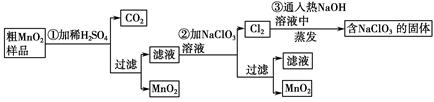
 ��
��
 ��Һ��
��Һ�� ��Һ�����ԣ�������ƽ��ԭ���ͱ�Ҫ�����ֽ�������C������3mol/L��
��Һ�����ԣ�������ƽ��ԭ���ͱ�Ҫ�����ֽ�������C������3mol/L��