��Ŀ����
��ͼ������Fe����ʯī������1 L����NaCl��Һ�С�����˵����ȷ���ǣ� ��
| A��M�Ӹ�����N��������������������������Ϊ22.4 L(��״��)ʱ������1 mol NaOH |
| B��M�Ӹ�����N������������Һ�е��˷�̪��Һ��C�缫��Χ��Һ��� |
| C��M�Ӹ�����N�������������ձ�����Һ����1 L CuSO4��Һ����Ӧһ��ʱ����ձ��в�����ɫ���� |
| D��M�ӵ�Դ������N�ӵ�Դ��������C�缫����Cu�缫���������Һ����CuSO4��Һ�����ʵ�������϶�ͭ |
A
�������������A��M�Ӹ�����N���������ܵ�ⷽ��ʽ�ǣ�2NaCl��2H2O Cl2����H2����2NaOH��������������������Ϊ��״��22.4 Lʱ��ÿ���缫����0.5mol�����壬��������1 mol NaOH����ȷ��B��M�Ӹ�����N������������������Fe�缫H+���Ϸŵ磬���Ը��������Һ�Լ��ԣ�����Һ�е��˷�̪��Һ��Fe�缫��Χ��Һ���,����C��M�Ӹ�����N�������������ձ�����Һ����1 L CuSO4��Һ�������ܷ���ʽ�ǣ�2CuSO4��2H2O
Cl2����H2����2NaOH��������������������Ϊ��״��22.4 Lʱ��ÿ���缫����0.5mol�����壬��������1 mol NaOH����ȷ��B��M�Ӹ�����N������������������Fe�缫H+���Ϸŵ磬���Ը��������Һ�Լ��ԣ�����Һ�е��˷�̪��Һ��Fe�缫��Χ��Һ���,����C��M�Ӹ�����N�������������ձ�����Һ����1 L CuSO4��Һ�������ܷ���ʽ�ǣ�2CuSO4��2H2O 2Cu+ O2����2H2SO4,�ձ��в�����ɫ����������D��M�ӵ�Դ������N�ӵ�Դ��������C�缫����Cu�缫�����������ǻ��Ե缫Fe,�缫ʧȥ���ӣ�������Ӧ��Fe-2e-=Fe2+��������Cu�Ϸ�����Ӧ��Cu2++2e-=Cu����ʵ�������϶�ͭ������
2Cu+ O2����2H2SO4,�ձ��в�����ɫ����������D��M�ӵ�Դ������N�ӵ�Դ��������C�缫����Cu�缫�����������ǻ��Ե缫Fe,�缫ʧȥ���ӣ�������Ӧ��Fe-2e-=Fe2+��������Cu�Ϸ�����Ӧ��Cu2++2e-=Cu����ʵ�������϶�ͭ������
���㣺������ԭ����Ӧ�õ�֪ʶ��

 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�����к͵ζ��������кͷ�Ӧ������֪Ũ�ȵ���(���)���ⶨδ֪Ũ�ȵļ�(����)��ʵ�鷽����������Һ��pH�仯���жϵζ��յ�����ݡ�
(1)Ϊ��ȷ������ͼ���ڵζ���ʼʱ��________����Һ�ٶȿ����Կ�һ�㣬���Ժͼ�¼pH�ļ���ɴ�Щ�����ӽ�________ʱ����Һ�ٶ�Ӧ����һЩ������ÿ��һ�ξͲ���һ�Ρ�
(2)��ͼ��A��pH��Χʹ�õ�ָʾ����____________��C��pH��Χʹ�õ�ָʾ����________________��D����Ϊ________________��
(3)��0.1 032 mol��L��1��������Һ�ζ�δ֪Ũ�ȵ�����������Һ���ظ����ε�ʵ���������±���ʾ��
| ʵ����� | ����0.1 032 mol��L��1��������Һ�����/mL | ��������������Һ�����/mL |
| 1 | 28.84 | 25.00 |
| 2 | 27.83 | 25.00 |
| 3 | 27.85 | 25.00 |
������������Ƶ����ʵ���Ũ����________mol��L��1���������ζ������У����ζ�ǰ�ζ����¶˼����������ݣ��ζ���������ʧ����ⶨ�����________(�ƫ�ߡ�����ƫ�͡���Ӱ�족)��
(4)���й��������к͵ζ������еIJ�����ȷ����________(����ĸ)��
A���ü�ʽ�ζ�����ȡδ֪Ũ�ȵ��ռ���Һ
B���ζ��ܺ���ƿ�������ô�ʢ��Һ��ϴ
C���ζ���ʼ��ע����ƿ����Һ��ɫ�仯
D����ƿ�еĴ���Һ����������ȡ
�����������ʴ�йص�˵����ȷ����( )
| A��ͼa�У����뺣ˮ�е�����Խ�����˸�ʴԽ���� |
| B��ͼb�У�������M������Nʱ��Cu-Zn�Ͻ�ĸ�ʴ���ʼ�С |
| C��ͼc�У���ͨ����ʱZn��ʴ��������Zn�Ϸų����������Ҳ���� |
| D��ͼd�У�Zn-MnO2�ɵ���Էŵ縯ʴ��Ҫ����MnO2��������������� |
��ͼ��ʾ��װ���У�MΪ���˳��λ����֮ǰ�Ľ�����NΪʯī�������й��ڴ�װ�õ������У�����ȷ���ǣ� ��
| A��N��������ų� |
| B��M������N���� |
| C����ѧ��ת��Ϊ���ܵ�װ�� |
| D���������е���ͨ��������������M��N |
Ϊ̽��������������ʴԭ���������ͼ��ʾװ�ã������й�˵���д������ 
| A�������ĵ缫����ʽΪ��O2��2H2O��4e��===4OH�� |
| B����ʯī�缫�ij�Mg�缫�����Թ۲쵽�������� |
| C����������ˮ�м�������NaCl(s)���ɽϿ�ؿ������� |
| D�������缫��������O2����ʯī�缫��������O2��������ֵÿ� |
����(Ni-Cd)�ɳ�������ִ��������й㷺Ӧ�á���֪ij���ӵ�صĵ������ҺΪKOH��Һ����䡢�ŵ簴��ʽ���У�Cd��2NiOOH��2H2O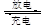 Cd(OH)2��2Ni(OH)2
Cd(OH)2��2Ni(OH)2
�йظõ�ص�˵����ȷ���ǣ� ��
| A���������ǻ�ѧ��ת��Ϊ���ܵĹ��� |
| B�����ʱ������Ӧ��Ni(OH)2��e����OH����NiOOH��H2O |
| C���ŵ�ʱ����������Һ�ļ�����ǿ |
| D���ŵ�ʱ�������Һ�е�OH���������ƶ� |
�ö��Ե缫���һ��Ũ�ȵ�CuSO4��Һʱ��ͨ��һ��ʱ��������õ���Һ�м���0.1mol Cu2 (OH)2CO3��ǡ�ûָ������ǰ��Ũ�Ⱥ�pH �������Ƕ�����̼���ܽ⣩�����������ת�Ƶĵ��ӵ����ʵ���Ϊ��
| A��0��4mol | B��0��5mol | C��0��6mol | D��0��8mol |
����ͼ��ʾ��ԭ����У����������ķ�Ӧ��
A��2H++2e�D H2�� H2�� | B��Cu�C2e�D Cu2+ Cu2+ |
C��Cu2++2e�D Cu Cu | D��Zn�C2e�D Zn2+ Zn2+ |
����ͼ��ʾװ�ý����й�ʵ�飬������������ȷ����
| A����װ������ԭ���ʱ��пΪ���� |
| B����װ������ԭ���ʱ�������Ϸ�����Ag+ +e��= Ag |
| C����װ������ͭƬ����ʱ���������� |
| D��ʵ��ͭƬ�������ʱ���ɽ�����п��������ͭƬ����������������������Ƭ���� |