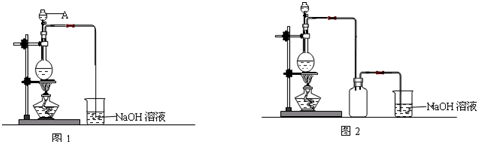
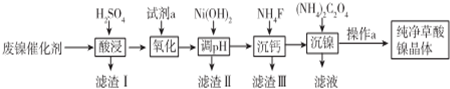
��Ŀ����
19����K+Ũ��Ϊ1mol•L-1��ij������Һ�У������ܺ����±��е����������ӣ�����֪H2SiO3Ϊ������ˮ�Ľ�״����������ʱ�ֽ�Ϊ���������Ag2CO3���������| ������ | Na+��Ag+��Ca2+��Ba2+ |
| ������ | NO3-��CO32-��SiO32-��SO42- |
| ��� | ʵ������ | ʵ���� |
| �� | �����Һ�м�������ϡ���� | ������ɫ��״�������ų���״����0.56L���� |
| �� | ����ķ�Ӧ���Һ���ˣ��Գ���ϴ�ӡ����������أ��������ù������� | ��������Ϊ2.4g |
| �� | ������Һ�еμ�BaCl2��Һ | ���������� |
��1��ʵ�����ȷ��һ�������ڵ�������Ag+��Mg2+��Ba2+��
��2��ʵ�����������������ӷ���ʽΪCO32-+2H+=H2O+CO2����
��3��ͨ��ʵ���ͱ�Ҫ���㣬NO3-�Ƿ���ڲ�һ����ѡ��ǡ���һ��������
��4���ж�Na+�Ƿ���ڣ�������������СŨ�ȴ��ڣ���Ũ������Ϊ0.3mol/L����������˵�����ɣ�-��
���� �����⡰��ҺΪ������Һ����֪����Һ�к��е�����һ���ܹ��������棻��ʵ����֪������Һ��һ������CO32-����Ũ��Ϊ$\frac{\frac{0.56L}{22.4L/mol}}{0.1L}$=0.25mol/L����һ��û��Ag+��Mg2+��Ba2+�������ɰ�ɫ�����ж���Һ��һ������SiO32-��������ӦSiO32-+2H+=H2SiO3����������ȷֽ����ɶ������裬��������Ϊ2.4gΪ������������������ݹ�ԭ���غ㣬SiO32-��Ũ��Ϊ��$\frac{\frac{2.4g}{60g/mol}}{0.1L}$=0.4mol/L����ʵ����֪��Һ�в���SO42-�����ݵ���غ�2c��CO32-��+2c��SiO32-��=2��0.25mol/L+2��0.4mol/L=1.3mol/L��0.5mol/L�������Һ��һ������Na+������Ũ������Ϊ0.8mol/L������ȷ��NO3-�Ƿ���ڣ��ݴ˽��н��
��� �⣺�����⡰��ҺΪ������Һ����֪����Һ�к��е�����һ���ܹ��������棻��ʵ����֪������Һ��һ������CO32-����Ũ��Ϊ$\frac{\frac{0.56L}{22.4L/mol}}{0.1L}$=0.25mol/L����һ��û��Ag+��Mg2+��Ba2+�������ɰ�ɫ�����ж���Һ��һ������SiO32-��������ӦSiO32-+2H+=H2SiO3����������ȷֽ����ɶ������裬��������Ϊ2.4gΪ������������������ݹ�ԭ���غ㣬SiO32-��Ũ��Ϊ��$\frac{\frac{2.4g}{60g/mol}}{0.1L}$=0.4mol/L����ʵ����֪��Һ�в���SO42-�����ݵ���غ�2c��CO32-��+2c��SiO32-��=2��0.25mol/L+2��0.4mol/L=1.3mol/L��1mol/L�������Һ��һ������Na+������Ũ������Ϊ0.8mol/L������ȷ��NO3-�Ƿ���ڣ�
��1����ʵ����֪����������ϡ�������ɰ�ɫ�������ڱ�״���·ų�0.56L���壬�����Һ��һ������CO32-��SiO32-����һ��û��Ag+��Mg2+��Ba2+��
�ʴ�Ϊ��Ag+��Mg2+��Ba2+��
��2��ʵ�����������������ӷ���ʽΪCO32-+2H+=H2O+CO2����
�ʴ�Ϊ��CO32-+2H+=H2O+CO2����
��3�����ݷ�����֪����Һ�в�һ��������������ӣ�
�ʴ�Ϊ����һ����
��4���������ϼ����֪������ȷ��NO3-��c��CO32-��=0.25mol/L��c��SiO32-��=0.4mol/L��һ����������������ӣ�����c��SO42-��=0�����ݵ���غ�2c��CO32-+��+2c��SiO32-��=2��0.25mol/L+2��0.4mol/L=1.3mol/L��1mol/L�������Һ��һ������Na+������Ũ������Ϊ0.3mol/L��
�ʴ�Ϊ�����ڣ���Ũ������Ϊ0.3mol/L��
���� ���⿼����ۺϣ��漰���ӹ��桢���Ӽ��鼰������ʵ���Ũ�ȵļ���ȣ�ע�ظ�Ƶ����Ŀ��飬��Ŀ�Ѷ��еȣ��������ӷ�Ӧ��Ӧ�������������ӵļ��鷽��Ϊ���Ĺؼ���ע�����غ��ж�Na+�Ƿ����Ϊ�������ѵ㡢�״��㣮

 �ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д�
�ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д� ��Ԫ������ĩ��ϵ�д�
��Ԫ������ĩ��ϵ�д���2FeCl3+2KI�T2FeCl2+2KCl+I2��
��2KMnO4+16HCl�T2MnCl2+2KCl+5Cl2+8H2O
��ij����Һ�к���Fe2+��I-��Cl-��Ҫ������ȥI-����Ӱ��Fe2+��Cl-���ɼ�����Լ��ǣ�������
| A�� | Cl2 | B�� | KMnO4 | C�� | FeCl3 | D�� | HCl |
| t�� | 700 | 800 | 830 | 1000 | 1200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK=$\frac{c��CO��c��{H}_{2}O��}{c��C{O}_{2}��c��{H}_{2}��}$��
��2���÷�ӦΪ���ȷ�Ӧ��ѡ�����ȡ����ȣ���
��3�����жϸ÷�Ӧ�Ƿ�ﵽ��ѧƽ��״̬��������bc����ѡ���÷֣���
a��������ѹǿ���� b����������� c��CO������
c��������H2��=������H2O�� d��c��CO2��=c��CO��
��4��ij�¶��£�ƽ��Ũ�ȷ�����ʽ��c��CO2��•c��H2��=c��CO��•c��H2O�������жϴ�ʱ���¶�Ϊ830�森
��5��ij�¶���SO2��ת����Ӧ��2SO2��g��+O2��g��?2SO3��g��ƽ�ⳣ��K=532.4��
�������������ϵ�и����ʵ�Ũ�����±���
| ��ϵ | c��SO2�� mol/L | c��O2�� mol/L | c��SO3�� mol/L | Ũ���� |
| ��1�� | 0.0600 | 0.400 | 2.000 | ���� |
| ��2�� | 0.0960 | 0.300 | 0.500 | ���� |
| ��3�� | 0.0862 | 0.263 | 1.020 | ���� |
| A�� | ʵ������Cl2��MnO2+2H++2Cl-�TMn2++Cl2��+2H2O | |
| B�� | �ù�����ˮ���չ�ҵβ���е�SO2��2NH3•H2O+SO2=2NH4++SO32-+H2O | |
| C�� | ����Ƭ��ĥ������NaOH��Һ�У�2Al+2OH-=2AlO2-+H2�� | |
| D�� | ������Һ�봿����Һ��Ϻ����壺2C6H5OH+CO32-=2C6H5O-+CO2��+H2O |
| A�� | ��0��Ԫ���⣬������Ԫ�ص�����ϼ�����ֵ�϶����ڸ�Ԫ��������������� | |
| B�� | �����ڱ������Ԫ�����ڵ�����������ԭ�Ӻ�������� | |
| C�� | ����Ԫ��û�зǽ���Ԫ�� | |
| D�� | Ԫ�ص�ԭ������Խ����ԭ�Ӱ뾶ҲԽ�� |
| A�� | ����������ˮ�ķ�Ӧ��O2-+H2O=2OH- | |
| B�� | ��������ˮ�ķ�Ӧ��Na+H2O=Na++OH-+H2�� | |
| C�� | ������������ͭ��Һ�ķ�Ӧ��2Na+Cu2+=Cu+2Na+ | |
| D�� | ����������Һ��̼��������Һ�ķ�Ӧ��H++HCO3-=H2O+CO2�� |
| X | ||
| Y | Z |
��2��������֤��YԪ����ZԪ�صĵõ�������ǿ������BC��������ţ�
A���⻯��ˮ��Һ������ B������������Ӧ��ˮ���������
C����̬�⻯����ȶ��� D�����������ʱÿ��ԭ�ӵõ��ӵĶ���
��3���Ƚ�Y��Z�����Ӱ뾶��С�������ӷ��ű�ʾ��S2-��Cl-
��4��XԪ�ص�ij����̬�⻯������к���10�����ӣ��������ڼ����������ܹ���CuO��ԭΪCu��ͬʱ����һ�����壬�������ǿ����еijɷ�֮һ����Ӧ�Ļ�ѧ����ʽΪ3CuO+2NH3$\frac{\underline{\;\;��\;\;}}{\;}$3Cu+3H2O+N2������Ӧ����6.02��1022�����ӷ���ת�ƣ�����4g��CuO�μӷ�Ӧ��