��Ŀ����
7������ʵ��������Ⱥ�˳���˵����ȷ���ǣ��������ټ����Թ�ʱ���Ⱦ��ȼ��ȣ���ֲ����ȣ�
������ˮ���ռ�����ʱ�����Ƴ����ܺƾ��ƣ�
����ȡ��������ʱ���ȼ��װ�������Ժ�װҩƷ��
��ʹ�÷�Һ©��ǰ���ȼ���Ƿ�©ˮ��ʹ�ã�
����PH��ֽ������Һ����ʱ����������ˮ����ֽʪ��Ȼ����飻
��ʹ��������ƽʱ��Ҫ�ȵ���������
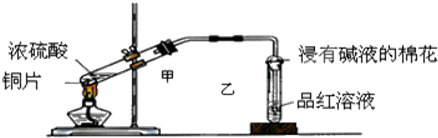
����H2��ԭCuOʵ��ʱ����ͨH2�����CuO����Ӧ��Ϻ��ȳ��ƾ��ƴ��Թ���ȴ��ֹͣͨH2��
| A�� | ȫ����ȷ | B�� | �������� | C�� | �������� | D�� | �������� |
���� ���Ⱦ��ȼ��ȣ���ֲ����ȿɷ�ֹ�Թֲܾ����ȶ�ը�ѣ�
��Ҫ�ȳ������ܣ�Ȼ��Ϩ��ƾ��ƣ�������������
���ȼ��װ�õ������ԣ���������ҩƷ���˷ѣ�
�ܷ�Һ©���Ļ������׳���©Һ����
��pH��ֽ��ˮ��ʪ�൱��ϡ���˱�����Һ��
�ޱ����ȵ��㣬�����������ʵ����
��H2��ԭCuOʱҪ��ͨH2���ž�ϵͳ�ڵĿ�����
��� �⣺�ټ����Թ�ʱ���Ⱦ��ȼ��ȣ���ֲ����ȿɷ�ֹ�Թֲܾ����ȶ�ը�ѣ��ʢ���ȷ��
������ˮ���ռ�����ʱ��Ҫ�ȳ������ܣ�Ȼ��Ϩ��ƾ��ƣ��������������ʢ���ȷ��
����ȡ��������ʱ��Ҫ�ȼ��װ�õ������ԣ���������ҩƷ���˷ѣ��ʢ���ȷ��
�ܷ�Һ©���Ļ������׳���©Һ����ʹ��ǰ�������Ƿ�©ˮ���ʢ���ȷ��
��pH��ֽ��ˮ��ʪ�൱��ϡ���˱�����Һ��ʹ��õ�pHֵ��ȷ���ʢݴ���
��ʹ��������ƽʱ�������ȵ��㣬�����������ʵ�����ʢ���ȷ��
��H2��ԭCuOʱҪ��ͨH2���ž�ϵͳ�ڵĿ��������ȼ�����ͨH2������ը��ʵ����Ϻ�����ͨH2�Դ��Թ���ȴ��������ֹͣͨH2���������������ʢ���ȷ��
��ѡD��
���� ���⿼�鳣��������ʹ�ã��ѶȲ�����Ϥ������������;��ʹ��ע��������ճ�����ѧʵ�����������ע�������ǽ���������Ĺؼ���

| Ԫ�� | �����Ϣ |
| X | Xһ�ֺ����ڿ���ʱ�������ᶨһЩ�������� |
| Y | Y��̬ԭ�ӵ�s���������P������������ |
| Z | Z���������ڵĵ��������а뾶��С |
| W | W�ĵ��ʱ���Ϊ����Ϣ�����Ĵ������������뵼����� |
| T | T�ж������������һ�ִ������������������¼���Ŵ��� ��Ѷ���ĵ�ԭ���� |
��2��T2+�ĵ����Ų�ʽΪ1s22s22p63s23p63d6��T�ĵ����ڸ�������Y���⻯�ﷴӦ���仯ѧ����ʽΪ3Fe+4H2O��g��$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2����ҵ����W����������X�ĵ��ʸ����·�Ӧ�Ƶ�W���ʵĴֲ�Ʒ���仯ѧ��Ӧ����ʽΪSiO2+2C $\frac{\underline{\;����\;}}{\;}$Si+2CO����
��3�������⻯��X2H2��H2Y2�зе�ϸߵ���H2O2�����߷е����ܴ��ԭ����H2O2����֮������������C2H2����֮��Ϊ���»�����
��4����25�桢101kPa�£���֪W�ȶ�����̬�⻯����Y����̬��������ȫȼ�գ��ָ���ԭ��״̬��ƽ��ÿ����4gW�ȶ�����̬�⻯�����190.0KJ����÷�Ӧ���Ȼ�ѧ����ʽSiH4��g��+2O2��g��=SiO2��s��+2H2O��l����H=-1520kJ/mol��
| A�� | H+H��H-H | B�� | H-Cl��H+Cl | ||
| C�� | Mg+2HCl=MgCl2+H2�� | D�� | H2SO4+2NaOH=Na2SO4+2H2O |
| A�� | �ں���FeBr2��FeI2����Һ�У�����ͨ��������I-��Br-��Fe2+ | |
| B�� | �ں���Ag+��Fe3+��Cu2+ ����Һ�м���п�ۣ�Ag+��Cu2+��Fe3+ | |
| C�� | �ں���NH4 +��H+��Al3+ ����Һ�У���μ���NaOH��Һ��H+��Al3+��NH4 +��Al��OH��3 | |
| D�� | �ں���Ca��OH��2��NaOH����Һ�У�����ͨ��CO2��NaOH��Ca��OH��2��Na2CO3��CaCO3 |
| A�� | �õ���ʽ��ʾ��������γɹ���Ϊ�� | |
| B�� | ��ˮ��Br-�ĵ���ʽΪ�� | |
| C�� | ��ˮ��ͨ������ʱ������Ӧ�����ӷ���ʽΪ��2Br-+Cl2=Br2+2Cl- | |
| D�� | Cl-�Ľṹʾ��ͼΪ�� |
| A�� | c��K+����c��NO3-����c��Cl-����c��Ag+����c��I-�� | B�� | c��K+����c��NO3-����c��Ag+����c��Cl-����c��I-�� | ||
| C�� | c��NO3-����c��K+����c��Ag+����c��Cl-����c��I-�� | D�� | c��K+����c��NO3-����c��Ag+��=��Cl-��+c��I-�� |
| A�� | X��ԭ��������Y��С | B�� | Xԭ�ӵ�������������Y�Ĵ� | ||
| C�� | X��Yԭ�ӵĵ��Ӳ������ | D�� | X��ԭ�Ӱ뾶��Y�Ĵ� |