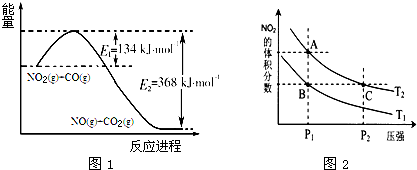
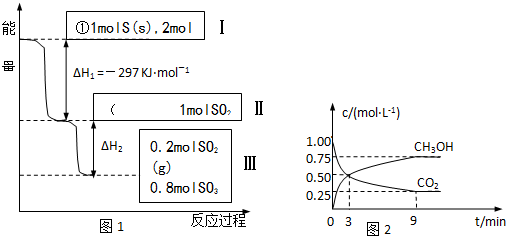
��Ŀ����
����˵�����ʾ������ȷ���ǣ�������
| A����C��ʯī��=C�����ʯ����H=+1.90 kJ?mol-1 ��֪���ʯ��ʯī�ȶ� | B����ϡ��Һ�У�H+��aq��+OH-��aq��=H2O��l����H=-57.3 kJ?mol-1��������0.5 mol H2SO4��Ũ�����뺬1 mol NaOH����Һ��ϣ��ų����ȴ���57.3 kJ | C������ı�ȼ����Ϊ��H=-890kJ?mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ�� CH4��g��+2O2��g��=CO2��g��+2H2O��g����H=-890 kJ?mol-1 | D����֪��H2��g��+F2��g��=2HF��g������H=-270 kJ?mol-1����1 mol������1 mol������Ӧ����2 molҺ̬������ų�������С��270 kJ |
������A�������Ȼ�ѧ����ʽ�ж������ߵͣ�����Խ��Խ���ȶ���
B��Ũ��������ˮ���ȣ�
C������ȼ���ȵĶ����жϣ�
D�������ΪҺ����ȣ�
B��Ũ��������ˮ���ȣ�
C������ȼ���ȵĶ����жϣ�
D�������ΪҺ����ȣ�
����⣺A����C��ʯī��=C�����ʯ����H=+1.90 kJ?mol-1 ��֪���ʯ�����ϸߣ���ʯī���ȶ�����A����
B��Ũ��������ˮ���ȣ����֪0.5 molŨ�����뺬1 mol NaOH����Һ��ϣ��ų����ȴ���57.3 kJ����B��ȷ��
C������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890 kJ?mol-1������Һ̬ˮ��Ϊ�ȶ�����C����
D�������ΪҺ����ȣ����Ȼ�ѧ����ʽ��֪1 mol������1 mol������Ӧ����2 molҺ̬������ų�����������270 kJ����D����
��ѡB��
B��Ũ��������ˮ���ȣ����֪0.5 molŨ�����뺬1 mol NaOH����Һ��ϣ��ų����ȴ���57.3 kJ����B��ȷ��
C������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890 kJ?mol-1������Һ̬ˮ��Ϊ�ȶ�����C����
D�������ΪҺ����ȣ����Ȼ�ѧ����ʽ��֪1 mol������1 mol������Ӧ����2 molҺ̬������ų�����������270 kJ����D����
��ѡB��
���������⿼�鷴Ӧ�����ʱ䣬������ѧ���ķ��������Ŀ��飬Ϊ�߿��������ͺ�Ƶ���㣬ע�����ȼ���ȡ��к��ȵĶ��壬�ѶȲ���ע����ػ���֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
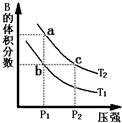 ���ڷ�ӦA��g��?2B��g����H��0�����¶�ΪT1��T2ʱ��ƽ����ϵ��B�����������ѹǿ�仯��������ͼ��ʾ���ش����и��⣮
���ڷ�ӦA��g��?2B��g����H��0�����¶�ΪT1��T2ʱ��ƽ����ϵ��B�����������ѹǿ�仯��������ͼ��ʾ���ش����и��⣮