��Ŀ����
1�� ��ҵ�ϳɰ������õ�����������һ�������·�Ӧ���ɵģ�������Ҫ�Ĺ�ҵԭ�ϣ���ش��������⣺
��ҵ�ϳɰ������õ�����������һ�������·�Ӧ���ɵģ�������Ҫ�Ĺ�ҵԭ�ϣ���ش��������⣺��1����Ԫ�������ڱ��е�λ���ǵ�2���ڢ�A�壻��ԭ�ӽṹʾ��ͼΪ
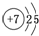 ��
����2��д�������ӵĵ���ʽ
 ��ָ�������л�ѧ���ǹ��ۼ�������Ӽ������ۼ�������
��ָ�������л�ѧ���ǹ��ۼ�������Ӽ������ۼ���������3��д�ɺϳɰ��Ļ�ѧ����ʽ��N2+3H2$\frac{\underline{\;���¡���ѹ\;}}{����}$2NH3��
�÷�Ӧ�Ƿ��ȷ�Ӧ������ͬ�����¡�1molN2��3molH2���������롰2molNH3���������ϸߵ���1molN2��3molH2��
��4����n��N2����n��H2��=1��3�Ļ��������뵽���������ܱ������У��ڴ����������·�����Ӧ������ڲ�ͬ�¶Ⱥ�ѹǿ�£������а������������ʱ���ϵ��ͼ��ʾ������ͼ�����3���еĻ�ѧ����ʽ�ɵó���
a��ѹǿ����ѧ��Ӧ���ʼӿ�
b���¶�����ѧƽ�����棨��������桱����Ӧ�����ƶ���
���� ��1����Ԫ����7��Ԫ�أ�ԭ�ӽṹʾ��ͼΪ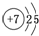 �����ݵ��Ӳ������������������������������������жϣ�
�����ݵ��Ӳ������������������������������������жϣ�
��2�������ӵķ���ʽΪNH3��������ԭ��֮��ͨ�����ۼ���ϣ��ݴ���д����ʽ��
��3�������������ڸ��¸�ѹ���������ºϳɰ����÷�Ӧ�Ƿ��ȷ�Ӧ����Ӧ����������������������������
��4������ͼ����ö�һ������������ȹ���ƽ�⣬��Ӧ���ʿ���ֵ���жϣ�
��� �⣺��1����Ԫ����7��Ԫ�أ�ԭ�ӽṹʾ��ͼΪ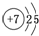 ����Ϊ���Ӳ����������������������������������������Ե�Ԫ�������ڱ��е�λ���ǵ�2���ڢ�A�壻�ʴ�Ϊ����2���ڢ�A�壻
����Ϊ���Ӳ����������������������������������������Ե�Ԫ�������ڱ��е�λ���ǵ�2���ڢ�A�壻�ʴ�Ϊ����2���ڢ�A�壻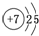 ��
��
��2�������ӵķ���ʽΪNH3��������ԭ��֮��ͨ�����ۼ���ϣ�����ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� �����ۼ���
�����ۼ���
��3�������������ڸ��¸�ѹ���������ºϳɰ�������ʽΪ��N2+3H2$\frac{\underline{\;���¡���ѹ\;}}{����}$2NH3����Ϊ��Ӧ�Ƿ��ȷ�Ӧ����Ӧ����������������������������������ͬ�����¡�1molN2��3molH2���������롰2molNH3���������ϸߵ���1molN2��3molH2���ʴ�Ϊ��N2+3H2$\frac{\underline{\;���¡���ѹ\;}}{����}$2NH3��1molN2��3molH2��
��4��a����ͼ���֪����400��ʱѹǿ����ȹգ����Է�Ӧ���ʴ�����ѹǿ����ѧ��Ӧ���ʼӿ죬�ʴ�Ϊ���ӿ죻
b����ͼ���֪����30MPaѹǿʱ���¶ȸߵİ����İٷֺ����ͣ�˵���¶�����ѧƽ�����淴Ӧ�����ƶ����ʴ�Ϊ���棻
���� ���⿼��ԭ�ӽṹ������ʽ����д�Լ���ѧƽ����ƶ������ڿ��鿼����ͼ��ķ��������Լ��Ի�ѧƽ������⣬�ѶȲ���

 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� |
��2����ЩԪ�ص�����������Ӧ��ˮ�����У�������ǿ�Ļ�����ķ���ʽ��HClO4��������ǿ�Ļ�����ĵ���ʽ
 �����γ��������������Ԫ����Al��
�����γ��������������Ԫ����Al����3���١��ڡ�������Ԫ�ص�ԭ�Ӱ뾶�ɴ�С��˳������ΪK��Na��Mg��
��4����Ԫ�ص��⻯����H2O�����⻯���ڳ�������ڷ�����Ӧ�Ļ�ѧ����ʽΪ2K+2H2O=2KOH+H2����������Һ��pH�� 7��
��5���õ���ʽ��ʾ�ٺ͢�Ԫ���γɻ�����Ĺ���
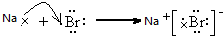 ��
�� | A�� | C2H5Cl��CH2=CH2 | B�� |  �� �� | ||
| C�� | CH��CH��CH2=CH2 | D�� | CH3COOH��CH3COOC2H5 |
| A�� | ��4He��һ������ | B�� | ��4He��һ������ | C�� | ��4He��ͬλ�� | D�� | ��4He��������ͬ |
| A�� | �٢� | B�� | �ۢ� | C�� | �٢� | D�� | �٢ۢ� |
| A�� | ������Һ����ˮ����ˮ | B�� | AgNO3��Һ��KBr��Һ��K2SO4��Һ | ||
| C�� | ����ϩ����ϩ����ʯ�� | D�� | KI��Һ��H2S��Һ��NaOH��Һ |
| A�� | ��500 mL������ƿ����250mL 0.1 mol/L��NaOH��Һ | |
| B�� | ����Ͳ�����ˮ���ռ��Ƶõ��������Ϊ50.28 mL | |
| C�� | ����²��22.4L N2������Ϊ28 g | |
| D�� | ��������ƽ�Ƶ�4.0 gNaOH���壬����1Lˮ���Ƶ�0.1mol/LNaOH��Һ |
 ��
��