��Ŀ����
(8��)����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
(1)��֪��N2(g)+O2(g)="2NO(g) " ��H=+180.5kJ/mol
N2(g)+3H2(g )
) 2NH3(g) ��H=��92.4kJ/mol
2NH3(g) ��H=��92.4kJ/mol
2H2(g)+O2(g)=2H2O(g) ��H=��483.6kJ/mol
��ɰ���������������һ�����������ˮ�������Ȼ�ѧ����ʽ��
4NH3��g��+5O2��g��==4NO��g��+6H2O��g������H= kJ��mol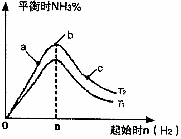
(2)ij����С���о����������������������£��ı���ʼ�����������ʵ����Է�ӦN2(g)+3H2(g)
 2NH3(g)��Ӱ�죮ʵ������ͼ��ʾ��(ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ���)
2NH3(g)��Ӱ�죮ʵ������ͼ��ʾ��(ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ���)
��ͼ����T1��T2�Ĺ�ϵ�ǣ�T1_______T2(����ڡ������ڡ������ڡ�����ȷ����)
�ڱȽ���a��b��c����������ƽ��״̬�У���Ӧ��N2��ת������͵���________(����ĸ)��
(3)��һ���¶Ⱥʹ����£���3.2mol H2��1.2molN2�����һ���ݻ�Ϊ2L���ܱ������з�����Ӧ����2minĩʱ��Ӧǡ�ô�ƽ�⣬��ʱ������0.8mol NH3������������µ�ƽ�ⳣ����(д��������̣��������С�����һλ)
��8�֣�
��1����905 ��2�֣�
��2���ٸ��ڣ�1�֣� ��b ��1�֣�
��3��0.4��L2/mol2�������̺ͽ��ȫ�Ե�4�֣���д��λ���۷֣�
����

 ��Դ�Ŀ����������������Ŀɳ����Է�չϢϢ��أ�
��Դ�Ŀ����������������Ŀɳ����Է�չϢϢ��أ� 2NH3
��H =-92.4 kJ/mol,��ʼ���ǽ�N2��H2�������20mol
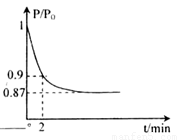
(�����1��1)����5L�ϳ�����.��ӦǰѹǿΪP0,��Ӧ������ѹǿ��P��ʾ����Ӧ������P/P0��ʱ��t�Ĺ�ϵ��ͼ��ʾ����ش��������⣺
2NH3
��H =-92.4 kJ/mol,��ʼ���ǽ�N2��H2�������20mol
(�����1��1)����5L�ϳ�����.��ӦǰѹǿΪP0,��Ӧ������ѹǿ��P��ʾ����Ӧ������P/P0��ʱ��t�Ĺ�ϵ��ͼ��ʾ����ش��������⣺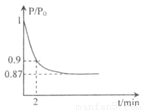
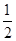 O2(g)=CO2(g)
��H2=b kJ��mol-1
O2(g)=CO2(g)
��H2=b kJ��mol-1 2NH3(g) ��H=-92.4kJ��mol-1����ʼ���ǽ�N2��H2�������20mol (�����1��1)����5L�ϳ����С���ӦǰѹǿΪP0����Ӧ������ѹǿ��P��ʾ����Ӧ������
2NH3(g) ��H=-92.4kJ��mol-1����ʼ���ǽ�N2��H2�������20mol (�����1��1)����5L�ϳ����С���ӦǰѹǿΪP0����Ӧ������ѹǿ��P��ʾ����Ӧ������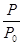 ��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
��ʱ��t�Ĺ�ϵ��ͼ��ʾ��