��Ŀ����
����Ŀ��ʵ�������Ҵ���ȡ��ϩ���õ�ʯ����Ҫ�ɷ�ΪCaC2��������CaS����ȡ��Ȳ��
��1��ʵ������ȡ��ϩ�Ļ�ѧ����ʽΪ_____________________________����������ȡ��ϩ�ķ���װ����_______����ѡ���ţ�

a b c d
��2���Ʊ���ϩ����ʱ�������¶ȹ��߶��۲쵽��Һ��ڣ�ͬʱ���ŵ��д̼�����ζ���������ɡ�����ϩ������Ʊ������У�Ũ����û�����ֵ�������________��
a ��ˮ�� b ��ˮ�� c ǿ������ d ����
�� �� �� ��
��ͬѧ�������ʵ��ȷ�������������������ϩ�Ͷ�������
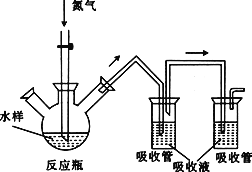
��ͼ����������������װ��ʢ�ŵ��Լ��ֱ��ǣ�������Ҫ����ѡ�����ţ���
��_______________����_______________����_______________����______________��
A Ʒ����Һ B NaOH��Һ C Ũ���� D ���Ը��������Һ
��3��ʵ�����Ʊ���Ȳ�Ļ�ѧ��Ӧ����ʽΪ_________________________________��ʵ��������ȲʱΪ�����ʯ��ˮ��Ӧ���ڼ��ң����÷�Һ©�����Ƶ����⣬ͨ��������_________����ˮ��Ϊ��ȥ��Ȳ�л��е������������ʣ��ɽ�����ͨ��___________��ѡ���ţ���
a����KMnO4��Һ b CCl4 c NaOH��Һ d CuSO4��Һ
���𰸡�CH3CH2OH![]() CH2=CH2��+H2O b d A B A D CaC2+2H2O��Ca(OH)2+HC��CH�� �ñ���ʳ��ˮ����ˮ cd
CH2=CH2��+H2O b d A B A D CaC2+2H2O��Ca(OH)2+HC��CH�� �ñ���ʳ��ˮ����ˮ cd
��������
��1��ʵ�������Ҵ���ȡ��ϩ���Ҵ���Ũ���������£����ȵ�170�淢����ȥ��Ӧ������ϩ��ˮ����Ӧ��Ӧʹ�¶�Ѹ�����µ�170�棬��ֹ����Ӧ������ʵ��ʱӦʹ���¶ȼƲ��뷴ӦҺ���Ʒ�Ӧ�¶ȣ�
��2���Ʊ���ϩ����ʱ��Ũ�������������ˮ�������ã�����������ˮ�Ժ�ǿ�����ԣ�ȷ�ϻ������������ϩ�Ͷ�������ʱ��Ӧע���������������ϩ�ļ��飻
��3��ʵ�����õ�ʯ��ˮ��Ӧ�Ʊ���Ȳ����ʯ�е�̼������ˮ��Ӧ�����������ƺ���Ȳ���Ʊ�ʱ�����÷�Һ©�����Ƶ��٣��ñ���ʳ��ˮ����ˮ���ﵽ���ƽ����Ȳ������Ŀ�ģ�������������������������Һ������ͭ��Һ��ȥ��Ȳ�л��е������������ʡ�
��1��ʵ�������Ҵ���ȡ��ϩ���Ҵ���Ũ���������£����ȵ�170�淢����ȥ��Ӧ������ϩ��ˮ����Ӧ�Ļ�ѧ����ʽΪCH3CH2OH ![]() CH2=CH2��+H2O���÷�Ӧ����ҺҺ���ȵķ�Ӧ����Ӧ��Ӧʹ�¶�Ѹ�����µ�170�棬��ֹ����Ӧ������ʵ��ʱӦʹ���¶ȼƲ��뷴ӦҺ���Ʒ�Ӧ�¶ȣ���ѡװ��b�Ʊ���ϩ���ʴ�Ϊ��CH3CH2OH
CH2=CH2��+H2O���÷�Ӧ����ҺҺ���ȵķ�Ӧ����Ӧ��Ӧʹ�¶�Ѹ�����µ�170�棬��ֹ����Ӧ������ʵ��ʱӦʹ���¶ȼƲ��뷴ӦҺ���Ʒ�Ӧ�¶ȣ���ѡװ��b�Ʊ���ϩ���ʴ�Ϊ��CH3CH2OH ![]() CH2=CH2��+H2O��b��
CH2=CH2��+H2O��b��
��2���Ʊ���ϩ����ʱ��Ũ�������������ˮ�������ã���Ũ���������ˮ�ԣ��¶ȹ���ʹ�Ҵ���ˮ̿�����۲쵽��Һ��ڣ���Ũ�������ǿ�����ԣ���������������ˮ̿�����ɵ�̼��Ӧ���ɶ�����̼�����������ˮ�����ŵ��̼�����ζ��Ũ����û�����ֵ����������ԣ�ȷ�ϻ������������ϩ�Ͷ�������ʱ��Ӧע���������������ϩ�ļ��飬�����ʢ��Ʒ����Һ�������������еĶ���������ʢ������������Һ����ȥ����������ʢ��Ʒ����Һ��ȷ�϶��������Ѿ���ȫ��ȥ�������������Ը��������Һ�������������е���ϩ���ʴ�Ϊ��d��A ��B��A��D��
��3��ʵ�����õ�ʯ��ˮ��Ӧ�Ʊ���Ȳ����ʯ����Ҫ�ɷ���̼���ƣ�̼������ˮ��Ӧ�����������ƺ���Ȳ����Ӧ�Ļ�ѧ����ʽΪCaC2��2H2O��Ca(OH)2��C2H2����ʵ��������ȲʱΪ�����ʯ��ˮ��Ӧ���ڼ��ң����÷�Һ©�����Ƶ����⣬ͨ�������ñ���ʳ��ˮ����ˮ���ﵽ���ƽ����Ȳ������Ŀ�ģ�������������������������Һ������ͭ��Һ��Ӧ�����ʣ�������ͨ������������Һ������ͭ��Һ��ȥ��Ȳ�л��е������������ʣ��ʴ�Ϊ��CaC2+2H2O��Ca(OH)2+HC��CH�����ñ���ʳ��ˮ����ˮ��cd��

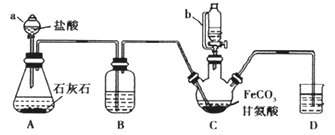
����Ŀ���ʰ�������[(NH2CH2COO)2Fe]��һ�ֲ���ǿ������ijѧϰС������FeCO3��ʰ���(NH2CH2COOH)�Ʊ��ʰ���������ʵ��װ������ͼ��ʾ���гֺͼ���������ʡ�ԣ���
�й������������±�
�ʰ��� | ������ | �ʰ������� |
������ˮ�������Ҵ� | ������ˮ���Ҵ� | ������ˮ���������Ҵ� |
���Ի����� | ǿ���ԡ�ǿ��ԭ�� |
ʵ����̣�
��ϳɣ�װ��C��ʢ��0.2 mol FeCO3��200 mL 1.0 mol��L1�ʰ�����Һ�����������ᡣʵ��ʱ���ȴ�����a�Ļ�������װ��C�п����ž����Ȳ����Ͻ��裬��ͨ������b��C�м�����������������Һ����p�ȵ�6���ң�ʹ��Ӧ���ַ�Ӧ��
����룺��Ӧ�������ˣ�����Һ��������Ũ����������ˮ�Ҵ������ˡ�ϴ�Ӳ����
�ش��������⣺
��1������C��������___________����a��ȣ�����b���ŵ���_______��
��2��װ��Bʢװ���Լ�Ϊ_____________��
��3���ϳɹ��̼���������������Ǵٽ�FeCO3�ܽ��______________��
��4����������������Һ����pH������6���ʰ������������½���ԭ��������ӷ���ʽ��ʾΪ________________��
��5������II��ϴ�Ӳ���Ϊ________��
��6�������Ʒ���Ƿ���Fe3+������Լ���_________��д��ѧ�����
��7����ʵ���Ƶ�15.3 g �ʰ�������(M=204g/mol)�����������_______����