��Ŀ����
����֪�ⶨ�к��ȵ�ʵ�鲽�����£�����ȡ50mL 0.25 mol/L���ᵹ��С�ձ��У������¶� ����ȡ50mL 0.55mol/L NaOH��Һ�������¶ȣ� �۽�NaOH��Һ����С�ձ��У���Ͼ��Ⱥ�������Һ�¶ȡ���ش�
��1��NaOH��Һ�Թ�����ԭ�� ��
��2������NaOH��Һ����ȷ������ (����ĸ����
A���ز������������� B��һ��Ѹ�ټ��� C�������μ���
��3��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������ ��
��4������Һ���ܶȾ�Ϊ1g��cm-3,�кͺ���Һ�ı�����c="4.18" J��(g����)-1�������ʵ����������к���Ϊ�������� ����д���÷�Ӧ���Ȼ�ѧ����ʽ_________
��5��������0.5 mol H2SO4��Ũ�����뺬1 mol NaOH����Һ��ϣ��ų������� ���С�ڡ��������ڡ����ڡ���57.3 kJ��ԭ�������������� ����
��ij�ռ���Ʒ�����������������õ����ʣ�Ϊ�˲ⶨ�䴿�ȣ��������²�����
A����250 mL������ƿ�ж������250 mL�ռ���Һ
B���ü�ʽ�ζ�����ȡ25.00 mL�ռ���Һ����ƿ�У������뼸�η�̪��ָʾ��
C������ƽ��ȷ��ȡ�ռ���ƷW g�����ձ���������ˮ�ܽ�
D�������ʵ���Ũ��ΪM�ı�������Һװ����ϴ�õ���ʽ�ζ����У�����Һ��ʹ��ʼ����ΪV1 mL
E.����ƿ�µ�һ�Ű�ֽ���ζ�����Һǡ���ɺ�ɫ��Ϊ��ɫʱ�����¶���ΪV2 mL
����գ�
��1����ȷ���������˳���� (����ĸ��ʾ����
��2���۲�ζ���Һ��ĸ߶�ʱӦע��
��3��E����IJ�������ƿ�µ�һ�Ű�ֽ�������� ��
��4��ijѧ��ʵ��ʱ����ƿ���ռ���Ʒ��Һϴ�ӣ�ʹ�ⶨ��Ũ��_________���ƫ�ߡ���ƫ�͡�����Ӱ�족����ԭ����
��5�����ռ���Ʒ���ȵļ���ʽΪ_________________________��
��1��ȷ�����ᱻ��ȫ�к� (2��B (3���û��β������������
(4��56.85 kJ��mol-1 , H2SO4��aq����2NaOH��aq����Na2SO4��aq��+2H2O ��H=-113.7kJ��mol-1
(5������ Ũ��������ˮ�ų�����
�𰸣���1��CABDE
(2���ζ���Ҫֱ����װҺ�����1��2 min����ܹ۲�Һ��߶ȣ�����ʱ������Һ��İ�Һ�漰�̶�����ͬһˮƽ���ϣ�����Ӧȷ��0.01 mL
(3��ʹ�ζ��յ���ɫ�仯�����ԣ����ڷֱ� (4��ƫ��
���Һ��Ʒմ����ƿ�ڱ�ʹ�ռ�����ʵ������ӣ����ñ�������ƫ���ռ�Ũ��ƫ��
(5��0.8��V2��V1��M/W��100%
(4��56.85 kJ��mol-1 , H2SO4��aq����2NaOH��aq����Na2SO4��aq��+2H2O ��H=-113.7kJ��mol-1
(5������ Ũ��������ˮ�ų�����
�𰸣���1��CABDE
(2���ζ���Ҫֱ����װҺ�����1��2 min����ܹ۲�Һ��߶ȣ�����ʱ������Һ��İ�Һ�漰�̶�����ͬһˮƽ���ϣ�����Ӧȷ��0.01 mL
(3��ʹ�ζ��յ���ɫ�仯�����ԣ����ڷֱ� (4��ƫ��
���Һ��Ʒմ����ƿ�ڱ�ʹ�ռ�����ʵ������ӣ����ñ�������ƫ���ռ�Ũ��ƫ��
(5��0.8��V2��V1��M/W��100%
��

��ϰ��ϵ�д�
�����Ŀ
 2SO3��g�� ��H="-197" kJ��mol-1����ͬһ�¶Ⱥ�ѹǿ�£���ij�ܱ�������ͨ��2 mol SO4��1 mol O2�ﵽƽ��ʱ����Ӧ�ų�������ΪQ1������һ��ͬ���ܱ�������ͨ��1 mol SO2��0.5 mol O2���ﵽƽ��ʱ�ų�������ΪQ2�����й�ϵ��ȷ���ǣ� ��
2SO3��g�� ��H="-197" kJ��mol-1����ͬһ�¶Ⱥ�ѹǿ�£���ij�ܱ�������ͨ��2 mol SO4��1 mol O2�ﵽƽ��ʱ����Ӧ�ų�������ΪQ1������һ��ͬ���ܱ�������ͨ��1 mol SO2��0.5 mol O2���ﵽƽ��ʱ�ų�������ΪQ2�����й�ϵ��ȷ���ǣ� ��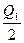
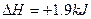 ��mol��1��֪���ʯ��ʯī�ȶ�
��mol��1��֪���ʯ��ʯī�ȶ�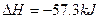 ��mol��1
��mol��1 O2��g����
O2��g����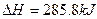 ��
�� mol��1
mol��1