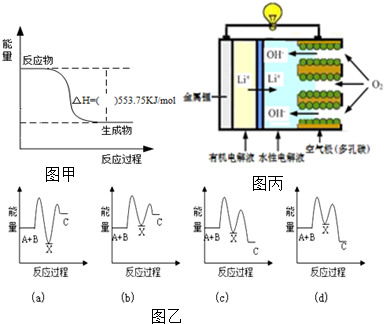
��Ŀ����
3������δ����õ���Դѡ����ȡ�����ij���ķ����кܶ࣬���ü״�������ˮ������Ӧ������������Ӧ����ʽ���£�CH3OH��g��+H2O��g��?CO2��g��+3H2��g����H��298K��=+49.4kJ/mol��һ�������£����ݻ�Ϊ2L�ĺ����ܱ������г���1mol CH3OH��g����3mol H2O��g����ʵ���ã��ﵽƽ��״̬ʱ����������19.76kJ������1����ƽ��ʱ��������ѹǿ�Ƿ�Ӧǰ��1.2����
��2���������µĸ÷�Ӧ��ƽ�ⳣ����0.11�����������λ��Ч���֣���
��3���������·�Ӧ��ƽ��״̬�������ǣ�����ţ�D��
A��v����CH3OH��=v����CO2�� B�����������ܶȲ���
C��c��CH3OH��=c��H2O�� D���������������ʵ�������
��4���״�ֱ��ȼ�ϵ�ص����Ϊ���ԣ�������ӦΪCH3OH-6e-+H2O=CO2��+6H+���õ�ص����������ѹΪ1.18V�������ܶ�E=5.92 kW•h•kg-1����ʽ���㣬���������λС�����������ܶ�=����������/ȼ��������1kW•h=3.6��106J��һ�����ӵĵ���Ϊ1.6��10-19C��NA=6.02��1023mol-1��
���� ��1������19.76KJ���ݷ���ʽ������ã���Ӧ��n��CH3OH��=$\frac{17.96kJ}{49.4kJ/mol}$=0.4mol����
CH3OH��g��+H2O��g���TCO2��g��+3H2��g����H��298K��=+49.4kJ/mol
��Ӧǰ��mol�� 1 3 0 0
��Ӧ�ˣ�mol�� 0.4 0.4 0.4 1.2
ƽ���mol�� 0.6 2.6 0.4 1.2
���º����£������ѹǿ֮�ȵ������ʵ���֮�ȣ��ݴ˼��㷴Ӧǰ��ѹǿ֮�ȣ�
��2����������ʽ�������ƽ��ʱ��Ũ�ȣ�K=$\frac{������Ũ����֮��}{��Ӧ��Ũ����֮��}$��
��3��ƽ��״̬ʱ���淴Ӧ������ȣ���Ӧ��ϵ�и����ʵ����ʵ������䡢���ʵ���Ũ�Ȳ��䡢�ٷֺ��������Լ��ɴ������һϵ�����������䣻
��4���״�ȼ�����Ե���У������ϼ״�ʧ���Ӻ�ˮ��Ӧ���ɶ�����̼�������ӣ�
����1Kg�״�����ĵ��ܣ���������ܶȹ�ʽ���㣮
��� �⣺��1������19.76KJ���ݷ���ʽ������ã���Ӧ��n��CH3OH��=$\frac{17.96kJ}{49.4kJ/mol}$=0.4mol����
CH3OH��g��+H2O��g���TCO2��g��+3H2��g����H��298K��=+49.4kJ/mol
��Ӧǰ��mol�� 1 3 0 0
��Ӧ�ˣ�mol�� 0.4 0.4 0.4 1.2
ƽ���mol�� 0.6 2.6 0.4 1.2
ƽ���������ѹǿ��ԭ����$\frac{��0.6+2.6+0.4+1.2��mol}{��1+3��mol}$=1.2��
�ʴ�Ϊ��1.2��
��2��K=$\frac{������Ũ����֮��}{��Ӧ��Ũ����֮��}$=$\frac{1��{2}^{3}��0.4}{0.6��2.6}$��0.11��
�ʴ�Ϊ��0.11��
��3��A����Ӧ������ͬ�������Ƿ�ﵽƽ��״̬������v����CH3OH��=v����CO2�������ܾݴ��ж�ƽ��״̬����A����
B��������䣬��Ӧ��������ﶼ�����壬�����ܶ���Զ���䣬���ܾݴ��ж�ƽ��״̬����B����
C���״���ˮ����Ũ����ȣ������ڼ�����������Ƿ�ƽ���أ����ܾݴ��ж�ƽ��״̬����C����
D����Ӧǰ�����������ͬ�����������ʵ�������˵�����淴Ӧ������ȣ���Ӧ�ﵽƽ��״̬����D��ȷ��
��ѡD��
��4�������ϼ״�ʧ���Ӻ�ˮ��Ӧ���ɶ�����̼�������ӣ��缫��ӦʽΪCH3OH-6e-+H2O=CO2��+6H+��
1Kg�״�����ĵ��ܣ�w=UIt=Uq=1.18��1000/32��6��6.02��1023��1.602��10-19J
=2.1699��107 J
=2.1337��107/3.6��106kW•h
=5.92 kW•h
�״�ֱ��ȼ�ϵ�������ܶ�E=5.92kW•h•kg-1��
�ʴ�Ϊ��CH3OH-6e-+H2O=CO2��+6H+��5.92��
���� ���⿼�黯ѧƽ����㡢ԭ���ԭ������ѧƽ��״̬�жϵ�֪ʶ�㣬Ϊ��Ƶ���㣬֪������ʽ���ڻ�ѧƽ������е�������ã�ֻ�з�Ӧǰ��ı��������������Ϊƽ��״̬�ж����ݣ��ѵ��ǣ�4������㣬�������ϴ���������֪ʶ���飬�ѵ�ϴ�

| A�� | ��CH3��3COH�����ƣ�2��2�����Ҵ� | B�� | ��������ģ�� | ||
| C�� | ��ȩ�Ľṹʽ��CH3CHO | D�� | ���Ȼ�̼���ӵĵ���ʽ�� |
| A�� | Fe | B�� | Mn | C�� | Co | D�� | Ni |
| A�� | SO2ͨ�����Եĸ��������Һ���Ϻ�ɫ��ȥ | |
| B�� | SO2ͨ��Ba��OH��2��Һ���в�����ɫ���� | |
| C�� | ���õ�Na2SO3��Һ�м����ữ��BaCl2��Һ������ɫ���� | |
| D�� | SO2ͨ����ˮ�������� |
���飺���麬Fe3+����Һ���Ƿ���Fe2+��
���飺���麬Fe2+����Һ���Ƿ���Fe3+��
�����Լ��������Լ�˳���ܴﵽʵ��Ŀ���ǣ�������
| �Լ� ѡ�� | ���� | ���� |
| A | ������ˮ��KSCN��Һ | NaOH��Һ |
| B | ����KmnO4��Һ | KSCN��Һ |
| C | KOH��Һ | ��ˮ |
| D | ��ˮ | ����KmnO4��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
MgSO4��s��+CO��g��?MgO��s��+CO2��g��+SO2��g����Ӧ�����вⶨ�IJ������ݼ�����
| ��Ӧʱ��/min | n��MgSO4��/mol | n��CO��/mol | n��CO2��/mol |
| 0 | 2.00 | 2.00 | 0 |
| 2 | 0.80 | ||
| 4 | 1.20 |
| A�� | ��Ӧ��0��2min�ڵ�ƽ������Ϊv��SO2��=0.6 mol•L-1•min-1 | |
| B�� | ��Ӧ��2��4min��������������ܶ�û�б仯 | |
| C�� | �������¶ȣ���Ӧ��ƽ�ⳣ����Ϊ1.00��������ӦΪ���ȷ�Ӧ | |
| D�� | ���������������䣬��ʼʱ�������г���1.00mol MgSO4��1.00molCO������ƽ��ʱn��CO2����0.60mol |
| A�� | ��֪NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l����H=-57.3 kJ•mol-1����40.0gNaOH��ϡ��Һ������ϡ������ȫ�кͣ��ų�������С��57.3kJ | |
| B�� | 2C��s��+2O2��g���T2CO2��g����H=akJ•mol-1��2C��s��+O2��g���T2CO��g����H=bkJ•mol-1����a��b | |
| C�� | ������ȼ����Ϊ285.5 kJ•mol-1����ˮ�ֽ���Ȼ�ѧ����ʽΪ��2H2O��l���T2H2��g��+O2��g������H=+285.5 kJ•mol-1 | |
| D�� | ��֪�����飨g�����춡�飨g����H��0������������춡���ȶ� |
| A�� | ʹ�ÿɽ������Ͼ۶�����̼���ܼ��ٰ�ɫ��Ⱦ | |
| B�� | �������о���������������Ϊ�����в����������Ķ������� | |
| C�� | ʵʩ��ú����������ú�ĵ硱�����ȼ�ϸ��칤�̣������ڱ������� | |
| D�� | ͨ����˵�������л��ϳɲ�����ָ���ϡ��ϳ���ά���ϳ��� |