ЬтФПФкШн
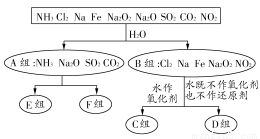
ЩшNAДњБэАЂЗќМгЕТТоГЃЪ§ЕФЪ§жЕЃЌЯТСаЫЕЗЈжае§ШЗЕФЪЧ ( )
ЂйГЃЮТГЃбЙЯТЃЌ17gМзЛљ(ЁЊ14CH3)ЫљКЌЕФжазгЪ§ЮЊ9NA
ЂкГЃЮТГЃбЙЯТЃЌ22.4 L NOЦјЬхЕФЗжзгЪ§аЁгкNA
Ђл64 gЭЗЂЩњбѕЛЏЛЙдЗДгІЃЌвЛЖЈЪЇШЅ2NAИіЕчзг
ЂмГЃЮТГЃбЙЯТЃЌ100 mL 0.5 molЁЄLЃ1ЕФввЫсШмвКжаЃЌввЫсЕФЗжзгЪ§ФПаЁгк0.05NA
ЂнБъзМзДПіЯТЃЌ22.4 LЖўТШМзЭщЫљКЌгаЕФЗжзгЪ§ЮЊNA
ЂоГЃЮТГЃбЙЯТЃЌ1 molКЄЦјКЌгаЕФКЫЭтЕчзгЪ§ЮЊNA
AЃЎЂйЂк BЃЎЂлЂм CЃЎЂкЂм DЃЎЂнЂо
C
ЁОНтЮіЁП1 molЁЊ14CH3жаЫљКЌжазгЕФЮяжЪЕФСПЮЊ(14Ѓ6) molЃЋ(1Ѓ1)ЁС3 molЃН8 mol,17 gЁЊ14CH3МДЮЊ1 molЃЌЂйВЛе§ШЗЃЛГЃЮТГЃбЙЯТЃЌ22.4 L NOЦјЬхЕФЮяжЪЕФСПаЁгк1 molЃЌдђЂке§ШЗЃЛЭЗЂЩњбѕЛЏЛЙдЗДгІЪБПЩЩњГЩЃЋ1МлЛђЃЋ2МлСНжжЛЏКЯЮяЃЌЫљвд1 mol CuПЩЪЇШЅ1 molЛђ2 molЕчзгЃЌЂлВЛе§ШЗЃЛгЩгкCH3COOHЮЊШѕЕчНтжЪЃЌПЩЗЂЩњЕчРыЃЌдђЂме§ШЗЃЛCH2Cl2дкБъзМзДПіЯТЮЊвКЬхЃЌЂнВЛе§ШЗЃЛКЄЦјЮЊЕЅдзгЗжзгЃЌ1 mol HeКЌга2 molЕчзгЃЌЂоВЛе§ШЗЁЃ

 зївЕИЈЕМЯЕСаД№АИ
зївЕИЈЕМЯЕСаД№АИ ЭЌВНбЇЕфвЛПЮЖрСЗЯЕСаД№АИ
ЭЌВНбЇЕфвЛПЮЖрСЗЯЕСаД№АИ ОЕфУмОэЯЕСаД№АИ
ОЕфУмОэЯЕСаД№АИ Н№ХЦПЮЬУСЗЯЕСаД№АИ
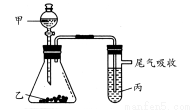
Н№ХЦПЮЬУСЗЯЕСаД№АИвбжЊСђЫсЁЂАБЫЎЕФУмЖШгыЫљМгЫЎСПЕФЙиЯЕШчЭМЫљЪОЃЌЯжгаСђЫсгыАБЫЎИївЛЗнЃЌЧыИљОнБэжааХЯЂЃЌЛиД№ЯТСаЮЪЬтЃК

| ШмжЪЕФЮяжЪЕФСП ХЈЖШ(molЁЄLЃ1) | ШмвКЕФУмЖШ(gЁЄcmЃ3) |
СђЫс | c1 | Іб1 |
АБЫЎ | c2 | Іб2 |
(1)БэжаСђЫсЕФжЪСПЗжЪ§ЮЊ (ВЛаДЕЅЮЛЃЌгУКЌc1ЁЂІб1ЕФДњЪ§ЪНБэЪО)ЁЃ
(2)ЮяжЪЕФСПХЈЖШЮЊc1 molЁЄLЃ1ЕФСђЫсгыЫЎЕШЬхЛ§ЛьКЯ(ЛьКЯКѓШмвКЬхЛ§БфЛЏКіТдВЛМЦ)ЃЌЫљЕУШмвКЕФЮяжЪЕФСПХЈЖШЮЊ molЁЄLЃ1ЁЃ
(3)ЮяжЪЕФСПХЈЖШЮЊc2 molЁЄLЃ1ЕФАБЫЎгы c2 molЁЄLЃ1ЕФАБЫЎЕШжЪСПЛьКЯЃЌЫљЕУШмвКЕФУмЖШ (ЬюЁАДѓгкЁБЁЂЁАаЁгкЁБЛђЁАЕШгкЁБЃЌЯТЭЌ)Іб2 gЁЄcmЃ3ЃЌЫљЕУШмвКЕФЮяжЪЕФСПХЈЖШ
c2 molЁЄLЃ1ЕФАБЫЎЕШжЪСПЛьКЯЃЌЫљЕУШмвКЕФУмЖШ (ЬюЁАДѓгкЁБЁЂЁАаЁгкЁБЛђЁАЕШгкЁБЃЌЯТЭЌ)Іб2 gЁЄcmЃ3ЃЌЫљЕУШмвКЕФЮяжЪЕФСПХЈЖШ  c2 molЁЄLЃ1(ЩшЛьКЯКѓШмвКЕФЬхЛ§БфЛЏКіТдВЛМЦ)ЁЃ
c2 molЁЄLЃ1(ЩшЛьКЯКѓШмвКЕФЬхЛ§БфЛЏКіТдВЛМЦ)ЁЃ