��Ŀ����
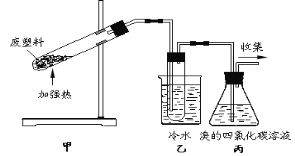
����Ŀ����1.2gij�л���M�����ܱ�������ȼ�գ�����ʵ�����������CO2��CO��ˮ��������ȼ�ղ�������ͨ��Ũ���ᡢ��ʯ�Һ����ȵ�����ͭ���Լ����������ҳ�ַ�Ӧ�������Ũ��������0.72g����ʯ������0.88g������ͭ����0.32g������˵���д�����ǣ� ��
A.M��ʵ��ʽΪCH2O
B.��Ҫ�õ�M�ķ���ʽ������Ҫ���M����Է������������ʵ���
C.��M����Է�������Ϊ60����M��һ��Ϊ����
D.ͨ���˴Ź������ɷ���M�еĹ�����
���𰸡�D
��������
Ũ�������ص�0.72gΪˮ��1.2g���л���ȼ������ˮ�����ʵ���Ϊ��![]() 0.04mol����ʯ�����ص�0.88gΪCO2�������������ʵ���Ϊ
0.04mol����ʯ�����ص�0.88gΪCO2�������������ʵ���Ϊ![]() 0.02mol��
0.02mol��
���л���ȼ�����ɵ�CO�����ʵ���Ϊx����
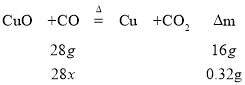
x=0.02mol��
1.2g�л���M�к���C�����ʵ���Ϊ��0.02mol+0.02mol=0.04mol������HԪ�ص����ʵ���Ϊ��0.04mol��2=0.08mol������C��HԪ�ص�������Ϊ��12g/mol��0.04mol+1g/mol��0.08mol=0.56g��1.2g��˵��M�к���OԪ�أ�����O�����ʵ���Ϊ![]() 0.04mol ��
0.04mol ��
A. M������C��H��O�����ʵ���֮��=0.04mol��0.08mol��0.04mol=1��2��1����M��ʵ��ʽΪCH2O����A��ȷ��
B. �Ѿ������M�����ʽ���ٲ��M����Է������������ʵ������ɼ����M�ķ���ʽ����B��ȷ��
C. ��M����Է�������Ϊ60����M�ķ���ʽΪ(CH2O)x��30x=60�����x=2��M�ķ���ʽΪC2H4O2��M����Ϊ���������������C��ȷ��
D. �ú�������ǿ���ȷ���л�������к��е��л�ԭ�ӻ��ţ��Ӷ���ȷ��M�����к��еĹ��������ͣ���D����
ѡD��