��Ŀ����
����Ŀ����ѧ��Ӧԭ���ڿ��к��������й㷺Ӧ�á�CO�����ںϳɼ״���һ���¶��£������Ϊ2 L���ܱ������м���CO��H2��������ӦCO(g)��2H2(g) ![]() CH3OH(g)���ﵽƽ����ø���ֵ�Ũ�����£�
CH3OH(g)���ﵽƽ����ø���ֵ�Ũ�����£�
���� | CO | H2 | CH3OH |
Ũ��/(mol��L��1) | 0.9 | 1.0 | 0.6 |
(1)��Ӧ�ﵽƽ��ʱ��CO��ת����Ϊ________��
(2)�÷�Ӧ��ƽ�ⳣ��K��________��
(3)���º��������£�����˵����Ӧ�Ѵﵽƽ��״̬����________(����ĸ)��
A��v��(CO)��2v��(H2)
B�����������ܶȲ���
C����������ƽ����Է�����������
D��CH3OH��CO��H2��Ũ�ȶ����ٷ����仯
(4)�����������ѹ����1 L����ﵽ��ƽ��ʱc(H2)��ȡֵ��Χ��__________��
(5)����������������䣬�ٳ���0.6 mol CO��0.4 mol CH3OH����ʱv��__v��(����>����<����������)��ƽ��_____________�ƶ���
���𰸡�40% ![]() (��0.67)��(L/mol) 2 CD 1.0 mol��L��1<c(H2)<2.0 mol��L��1 �� ��
(��0.67)��(L/mol) 2 CD 1.0 mol��L��1<c(H2)<2.0 mol��L��1 �� ��
��������
��1�����ݻ�ѧ�����ȣ��״���һ����̼�ļ�������1:1��Ҳ����˵����0.6mol/L�״���Ҫ����0.6 mol/L��![]() ����˷�Ӧǰһ����̼��Ũ��Ϊ1.5 mol/L����ת����Ϊ
����˷�Ӧǰһ����̼��Ũ��Ϊ1.5 mol/L����ת����Ϊ![]() ��
��
��2�����ݷ���ʽ��֪K�ı���ʽΪ![]() ���ٴ���ƽ��ʱ�����ʵ�ƽ��Ũ�ȿɵ�
���ٴ���ƽ��ʱ�����ʵ�ƽ��Ũ�ȿɵ�![]() ��
��
��3��A.���ݷ�Ӧ�Ļ�ѧ������֮��Ӧ����2v��(![]() )=v��(
)=v��(![]() )ʱ������˵����Ӧ�ﵽƽ��״̬��A�����
)ʱ������˵����Ӧ�ﵽƽ��״̬��A�����
B.��Ϊ����һ��������ϵ���������ʶ������壬�����ܶ��Ǻ㶨����ģ�B�����
C.��Ϊ�÷�Ӧ��һ����Ӧǰ��������������ȵķ�Ӧ����˵������ƽ����Է�����������ʱ��˵����Ӧ�Ѵ�ƽ�⣬C����ȷ��
D.���з�Ӧ����������Ũ�Ȳ������Ƿ�Ӧ�ﵽƽ���������D����ȷ��
��ѡCD��
��4�����ȸ���![]() ������ƽ�ⲻ��������ĸı���ƶ��������������С��һ��ʱ������Ũ�ȱ��2������2 mol/L������ƽ��Ҫ�ƶ�������������ԭ����֪�������С��ѹǿ����ʱ��ƽ��Ҫ��������������٣�ѹǿ��С���ķ�����У�Ҳ����������У��������Ҫ������һ���֣��������ϵ�2 mol/LҪ�٣�������Ȼ����1 mol/L��
������ƽ�ⲻ��������ĸı���ƶ��������������С��һ��ʱ������Ũ�ȱ��2������2 mol/L������ƽ��Ҫ�ƶ�������������ԭ����֪�������С��ѹǿ����ʱ��ƽ��Ҫ��������������٣�ѹǿ��С���ķ�����У�Ҳ����������У��������Ҫ������һ���֣��������ϵ�2 mol/LҪ�٣�������Ȼ����1 mol/L��
��5���ڶ����Ѿ�����˸÷�Ӧ��KֵΪ0.67�����¶�δ�ı�ʱKֵҲ����ı䣬���µļ״�Ũ�Ⱥ�һ����̼Ũ�ȴ���Ũ����Q��![]() �����ִ�ʱQ����K��ȣ����ƽ�ⲻ�ƶ���ƽ��û�ƶ�Ҳ����˵���淴Ӧ������Ȼ��ȡ�
�����ִ�ʱQ����K��ȣ����ƽ�ⲻ�ƶ���ƽ��û�ƶ�Ҳ����˵���淴Ӧ������Ȼ��ȡ�

 �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�����Ŀ����Ӧ�û�ѧ��Ӧԭ�������֪ʶ����������⣺
![]() ��֪NaCl���ܽ���Ϊ
��֪NaCl���ܽ���Ϊ![]() ����
����![]()
![]()
![]()
![]()
![]()
д������������ȼ�յ��Ȼ�ѧ����ʽ��__________��
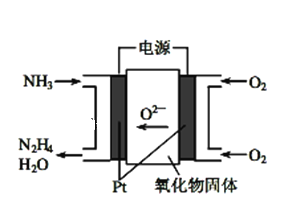
![]() һ�������£���
һ�������£���![]() �����ܱ������г���
�����ܱ������г���![]() ��
��![]() ������Ӧ��
������Ӧ��![]() ����ͼ��ʾΪ��Ӧ��ϵ��
����ͼ��ʾΪ��Ӧ��ϵ��![]() ��ƽ��ת�������¶ȵĹ�ϵ���ߡ���֪���¶�Ϊ
��ƽ��ת�������¶ȵĹ�ϵ���ߡ���֪���¶�Ϊ![]() �������£��÷�Ӧ
�������£��÷�Ӧ![]() �ﵽƽ��״̬��
�ﵽƽ��״̬��
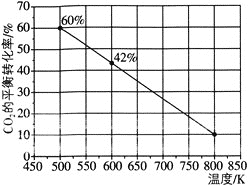
![]() �÷�Ӧ��________
�÷�Ӧ��________![]() ��������������������
��������������������![]() ��Ӧ��
��Ӧ��
![]() ��
��![]() ʱ�η�Ӧ����
ʱ�η�Ӧ����![]() Ϊ_________��
Ϊ_________��
![]() ���ij���
���ij���![]() ��
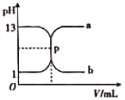
��![]() ��ͼ�е�����_________
��ͼ�е�����_________![]() ����
����![]() ��
��
![]() �����±����ݻش����⣺
�����±����ݻش����⣺
��1 ![]() ʱŨ��Ϊ
ʱŨ��Ϊ![]() ������Һ��pH
������Һ��pH
���� | NaClO |
|
pH |
|
|
��2 ![]() ʱ������ĵ���ƽ�ⳣ��
ʱ������ĵ���ƽ�ⳣ��
|
| |
|
|
|
|
|
|
![]() ���ݱ�1�ܲ����жϳ�
���ݱ�1�ܲ����жϳ�![]() ��HClO����ǿ����_____________
��HClO����ǿ����_____________![]() ������������������
������������������![]() ��
��
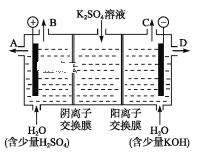
![]() ��Һ������Ũ���ɴ�С��˳��Ϊ__________��
��Һ������Ũ���ɴ�С��˳��Ϊ__________��
![]() ��Һ��
��Һ��![]() ��Һ��Ӧ�����ӷ���ʽΪ________��
��Һ��Ӧ�����ӷ���ʽΪ________��
![]() ��֪��
��֪��![]() ʱ��
ʱ��![]() ��
��![]() ��AgClΪ��ɫ������
��AgClΪ��ɫ������![]() Ϊש��ɫ������
Ϊש��ɫ������![]() ʱ����
ʱ����![]() ��
��![]() Ũ�Ⱦ�Ϊ
Ũ�Ⱦ�Ϊ![]() �Ļ����Һ����μ���
�Ļ����Һ����μ���![]() ��Һ�������Ҳ��Ͻ��裬ʵ������Ϊ__________��
��Һ�������Ҳ��Ͻ��裬ʵ������Ϊ__________��