��Ŀ����
����Ŀ����д��N2�ĵ���ʽ__________��������ʵ���˹��̵�����__________��
A������ B���ڼ�ѹ���µ�������ʹ�����еĵ���Һ��
C������ D���ϳɰ�����
��ʵ����ͨ���ü�����ʯ�����Ȼ�粒���ķ�������ȡ������
д���÷�Ӧ�Ļ�ѧ����ʽ________________________________________��
ʵ������ȡ�����ķ����ж��֣������װ�ú�ѡ�õ��Լ��д������__________��

�ǰ�����ʹʪ��ĺ�ɫʯ����ֽ������ԭ���û�ѧ�����ʾ��
____________________________________________________________
������װ������һ��ʱ�䰱����ͨ�������ͬʱ�����ȵIJ�˿������ װ�õ���ƿ�ڣ���ƿ�в��������ɵ������ǣ�__________��
A�� ![]() B��
B�� ![]() C��
C�� ![]() D��
D�� ![]()
д����װ���а��������Ļ�ѧ����ʽ��_____________________��
����֪![]() �����³�ѹ�£���һ�ܱ������н�
�����³�ѹ�£���һ�ܱ������н�![]() ��
��![]() ��ַ�Ӧ��ʣ����������Ϊ__________
��ַ�Ӧ��ʣ����������Ϊ__________ ![]() ��
��
���ڱ�״���£� ![]() ˮ�п��ܽ�
ˮ�п��ܽ�![]() ��������Һ���ܶ�Ϊ
��������Һ���ܶ�Ϊ![]() ����ˮ�����ʵ���Ũ��Ϊ__________
����ˮ�����ʵ���Ũ��Ϊ__________ ![]() ������һλС������
������һλС������
���𰸡� ![]() D�� Ca(OH)2+2NH4Cl
D�� Ca(OH)2+2NH4Cl![]() CaCl2+2NH3��+2H2O AC NH3+ H2O
CaCl2+2NH3��+2H2O AC NH3+ H2O![]() NH3�� H2O
NH3�� H2O![]() NH4++ OH�� A 4NH3+5O2
NH4++ OH�� A 4NH3+5O2![]() 4NO+6H2O 5 18.4
4NO+6H2O 5 18.4
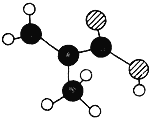
����������1����ԭ�Ӽ��γ����Թ��õ��Ӷԣ�����ʽΪ![]() ��A�����磬�����˹��̵���ѡ��A����B���ڼ�ѹ���µ�������ʹ�����еĵ���Һ�������ǹ̵�����Һ�������õ�����ѡ��B������C����������������̵���ѡ��C����D���ϳɰ����䣬�����˹��̵���ѡ��D��ȷ����ѡD����ʵ����ͨ���ü�����ʯ�����Ȼ�粒���ķ�������ȡ�������÷�Ӧ�Ļ�ѧ����ʽΪ��Ca(OH)2+2NH4Cl
��A�����磬�����˹��̵���ѡ��A����B���ڼ�ѹ���µ�������ʹ�����еĵ���Һ�������ǹ̵�����Һ�������õ�����ѡ��B������C����������������̵���ѡ��C����D���ϳɰ����䣬�����˹��̵���ѡ��D��ȷ����ѡD����ʵ����ͨ���ü�����ʯ�����Ȼ�粒���ķ�������ȡ�������÷�Ӧ�Ļ�ѧ����ʽΪ��Ca(OH)2+2NH4Cl![]() CaCl2+2NH3��+2H2O��A���Ȼ�粒����������ȡ������ѡ��A��ѡ��B��Ũ��ˮ�������ƿ�����ȡ��������Ӧװ��Ҳ��ȷ��ѡ��B��ѡ��C�����Թ�Ӧ����������б������Cװ�ô���ѡ��C��ѡ��D��Ũ��ˮ������ȡ�������Լ���װ�ö���ȷ��ѡ��D��ѡ����ѡAC����ʪ��ĺ�ɫ��ʯ����ֽ������ԭ���ǰ�����ˮ��Ӧ������һˮ�ϰ���һˮ�ϰ���������������ӣ���Ӧ�ķ���ʽΪ��NH3+ H2O
CaCl2+2NH3��+2H2O��A���Ȼ�粒����������ȡ������ѡ��A��ѡ��B��Ũ��ˮ�������ƿ�����ȡ��������Ӧװ��Ҳ��ȷ��ѡ��B��ѡ��C�����Թ�Ӧ����������б������Cװ�ô���ѡ��C��ѡ��D��Ũ��ˮ������ȡ�������Լ���װ�ö���ȷ��ѡ��D��ѡ����ѡAC����ʪ��ĺ�ɫ��ʯ����ֽ������ԭ���ǰ�����ˮ��Ӧ������һˮ�ϰ���һˮ�ϰ���������������ӣ���Ӧ�ķ���ʽΪ��NH3+ H2O![]() NH3�� H2O
NH3�� H2O![]() NH4++ OH�����Ȣ۰���������������������һ��������ˮ��һ�������ܹ����������ɶ�������������������ˮ��Ӧ����һ�����������ᣬ�����ܹ��백����Ӧ��������泥����Բ��������ɵ�����������ѡA�������������ķ�Ӧ�ķ���ʽΪ4NH3+5O2
NH4++ OH�����Ȣ۰���������������������һ��������ˮ��һ�������ܹ����������ɶ�������������������ˮ��Ӧ����һ�����������ᣬ�����ܹ��백����Ӧ��������泥����Բ��������ɵ�����������ѡA�������������ķ�Ӧ�ķ���ʽΪ4NH3+5O2![]() 4NO+6H2O����5��15ml Cl2��40ml NH3��Ӧ�����ݷ�Ӧ����ʽ3Cl2+2NH3=N2+6HCl��15mL������Ӧ���İ���10mL����������5mL������30mL�Ȼ��⣻���ڰ������Ȼ��ⷴӦ�����Ȼ�泥�ʣ��İ���ǡ�������ɵ��Ȼ�立�Ӧ��ʣ�����ʣ��������ǵ��������Ϊ5mL����6�����������ʵ���Ϊ��
4NO+6H2O����5��15ml Cl2��40ml NH3��Ӧ�����ݷ�Ӧ����ʽ3Cl2+2NH3=N2+6HCl��15mL������Ӧ���İ���10mL����������5mL������30mL�Ȼ��⣻���ڰ������Ȼ��ⷴӦ�����Ȼ�泥�ʣ��İ���ǡ�������ɵ��Ȼ�立�Ӧ��ʣ�����ʣ��������ǵ��������Ϊ5mL����6�����������ʵ���Ϊ�� ![]() mol=31.25mol����Һ������Ϊ��1000g+17��31.25g=1531.25g����ˮ��Һ�����Ϊ��
mol=31.25mol����Һ������Ϊ��1000g+17��31.25g=1531.25g����ˮ��Һ�����Ϊ�� ![]() ��1.70L��
��1.70L��
����ˮ��Ũ��Ϊ�� ![]() ��18.4mol/L��
��18.4mol/L��