��Ŀ����
20��һ���¶��£���3mol A�����1mol B����ͨ��һ�ܱ������У��������·�Ӧ��3A��g��+B��g��?xC��g��������д���пհף���1������������̶�Ϊ2L����Ӧ2minʱ���ʣ��0.6mol B��C��Ũ��Ϊ0.4mol/L��
��x=2��
��4min��C��Ũ��С��0.8mol/L ������ڡ��������ڡ���С�ڡ�����
����֪ƽ��������C���������Ϊ25%����A��ת����Ϊ40%��
�ܸı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ���������ԭƽ����ȣ���ʼ������������ʵ����ʵ����ֱ�Ϊa��b��c������֮��Ӧ������Ĺ�ϵʽΪn��A��+1.5n��C��=3��n��B��+0.5n��C��=1��
��2����ά������ѹǿ���䣬�ı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ�����ԭƽ���2������Ӧ����6mol A�����2mol B���壮
���� ��1��һ���¶��£���3molA�����1molB����ͨ��һ�ܱ������У�����������̶�Ϊ 2L����Ӧ2minʱ���ʣ��0.6mol B��C��Ũ��Ϊ0.4mol/L����
3A��g��+B��g��?x C��g��
��ʼ����mol����3 1 0
�仯����mol����1.2 0.4 0.4x
2minʱ��mol����1.8 0.6 0.4x
�ٽ��C��Ũ�ȼ���x��
�ں�2min������С��ǰ2min�ڷ�Ӧ���ʣ��ʺ�2min��C��Ũ�ȱ仯��С��0.4mol/L��
����ƽ��ʱת����BΪymol����ʾ��ƽ��ʱ����ֵ����ʵ������ٸ���C���������Ϊ25%�з��̼�����
�ܸı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ���������ԭƽ����ȣ����º����£���Ӧǰ�������������ȣ�����ѧ������ת�����������n��A��=3mol��n��B��=1mol��
��2����ά������ѹǿ���䣬�ı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ�����ԭƽ���2������ԭƽ���Ч����Ӧ��ת������ȣ�Ӧ����A��B�����ʵ���֮�����ʼ����������Ϊԭ����2����
��� �⣺��1��һ���¶��£���3molA�����1molB����ͨ��һ�ܱ������У�����������̶�Ϊ 2L����Ӧ1minʱ���ʣ��0.6mol B��C��Ũ��Ϊ0.4mol/L����
3A��g��+B��g��?x C��g��
��ʼ����mol����3 1 0
�仯����mol����1.2 0.4 0.4x
1minʱ��mol����1.8 0.6 0.4x
��$\frac{0.4xmol}{2L}$=0.4mol/L����x=2���ʴ�Ϊ��2��
�ں�1min������С��ǰ1min�ڷ�Ӧ���ʣ��ʺ�1min��C��Ũ�ȱ仯��С��0.4mol/L����2min�ﵽƽ�⣬ƽ��ʱC��Ũ��С��0.8mol/L���ʴ�Ϊ��С�ڣ�
����ƽ��ʱת����BΪymol����
3A��g��+B��g��?2 C��g��
��ʼ����mol����3 1 0
�仯����mol����3y y 2y
2minʱ��mol����3-3y 1-y 2y
����$\frac{2y}{4-2y}$=25%�����y=0.4����A��ת����Ϊ$\frac{0.4mol��3}{3mol}$��100%=40%��
�ʴ�Ϊ��40%��
�ܸı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ���������ԭƽ����ȣ����º����£���Ӧǰ�������������ȣ�����ѧ������ת�����������n��A��=3mol��n��B��=1mol����n��A��+1.5n��C��=3��n��B��+0.5n��C��=1��
�ʴ�Ϊ��n��A��+1.5n��C��=3��n��B��+0.5n��C��=1��
��2����ά������ѹǿ���䣬�ı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ�����ԭƽ���2������ԭƽ���Ч����Ӧ��ת������ȣ�Ӧ����A��B�����ʵ���֮�����ʼ����������Ϊԭ����2������Ӧ����6molA��2molB��
�ʴ�Ϊ��6��2��
���� ���⿼�黯ѧƽ��ļ��㣬��Ŀ�Ѷ��еȣ���1���Т�ע����ݷ�Ӧ���ʷ���ǰ����2min��C��Ũ�ȱ仯��
���״���Ϊ��2�����ؼ��ǶԵ�Чƽ������⣮

| ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
| n��O2��/mol | 0.7 | 0.4 | 0.3 | x | x | x |
��1����O2��ʾ��0��2s�ڸ÷�Ӧ��ƽ����Ӧ����Ϊ0.4mol•L-1•s-1��
��2��O2��ƽ��Ũ��c��O2��=0.5mol•L-1��
��3����÷�Ӧ�ﵽƽ��ʱSO2��ת������90%���ðٷ�����ʾ����
��4������ƽ���������5%ͨ�������BaCl2��Һ�����ɳ���10.5�ˣ�����������һλС������
| A�� | ������Һ�е�SO42-����Һ$\stackrel{BaCl_{2}��Һ}{��}$ ��ɫ����$\stackrel{HCl��Һ}{��}$ ��ɫ���� | |
| B�� | ������Һ�е�Cl-����Һ$\stackrel{ϡH_{2}SO_{4}}{��}$����$\stackrel{AgNO_{3}��Һ}{��}$ ��ɫ���� | |
| C�� | ������Һ�е�Fe2+����Һ$\stackrel{��ˮ}{��}$����������$\stackrel{KSCN��Һ}{��}$Ѫ��ɫ��Һ | |
| D�� | ������Һ�е�NH4+����Һ$��_{����}^{NaOH��Һ}$�����ݳ�$��_{ʯ����ֽ}^{ʪ��ĺ�ɫ}$ ��ֽ���� |
| A�� | �����ǡ����ǡ����Ǻ͵��۶��ܷ���ˮ�ⷴӦ | |
| B�� | ������֬�ڼ��������µ�ˮ�⣬�����Ƹ��ͺͷ��� | |
| C�� | ���ࡢ��֬�������ʶ�����C��H��O����Ԫ����ɵĸ߷��ӻ����� | |
| D�� | ���ۺ���ά�صķ��������ͬ������֮�以Ϊͬ���칹�� |
 ʵ��������500mL 0.2mol•L-1��Na2SO4��Һ��ʵ����������У�
ʵ��������500mL 0.2mol•L-1��Na2SO4��Һ��ʵ����������У�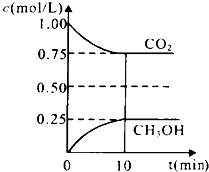 ̼��̼�Ļ������������������е�Ӧ�÷dz��㷺�����ᳫ���������ѳɳ����Ľ��죬����̼�������ֻ��һ�����룬����һ��ֵ���ڴ����µ����ʽ����һ���¶��µ�2L�̶��ݻ����ܱ������У�ͨ��2molCO2��3mol H2�������ķ�ӦΪ��CO2��g��+3H2��g��CH3OH��g��+H2O��g������H=-a kJ•mol-1��a��0�������CO2��g����CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��
̼��̼�Ļ������������������е�Ӧ�÷dz��㷺�����ᳫ���������ѳɳ����Ľ��죬����̼�������ֻ��һ�����룬����һ��ֵ���ڴ����µ����ʽ����һ���¶��µ�2L�̶��ݻ����ܱ������У�ͨ��2molCO2��3mol H2�������ķ�ӦΪ��CO2��g��+3H2��g��CH3OH��g��+H2O��g������H=-a kJ•mol-1��a��0�������CO2��g����CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��
 ��������ͼ3��ʾװ����ȡ����Һ����Ҫ�ɷ�ΪNaClO��������A��Ϊ������B��Ϊ�������������IJ���ΪCl2���ѧʽ����
��������ͼ3��ʾװ����ȡ����Һ����Ҫ�ɷ�ΪNaClO��������A��Ϊ������B��Ϊ�������������IJ���ΪCl2���ѧʽ����