��Ŀ����
8���̷���FeSO4•7H2O��������ȱ����ƶѪ����Чҩ��ijѧУ�Ļ�ѧ��ȤС���ͬѧ���̷����������µ�̽������һ��FeSO4•7H2O���Ʊ�
�û�ѧ��ȤС���ͬѧ��ʵ����ͨ������ʵ���ɷ���м������������ͭ�������������ʣ��Ʊ�FeSO4•7H2O���壺
�ٽ�5%Na2CO3��Һ���뵽ʢ��һ��������м���ձ��У����������ӣ�����������ȥNa2CO3��Һ��Ȼ����м��ˮϴ��2��3�飮
����ϴ�ӹ��ķ���м�м��������ϡ���ᣬ�����¶���50��80��֮������м�ľ���
�۳��ȹ��ˣ�����Һת�뵽�ܱ������У����á���ȴ�ᾧ��
�ܴ��ᾧ��Ϻ��˳����壬��������ˮϴ��2��3�Σ�������ֽ���������ɣ�
�ݽ��Ƶõ�FeSO4•7H2O�������һ��С���ƿ�У��ܱձ��森
��ش��������⣺
��1��ʵ�鲽��ٵ�Ŀ���dz����ۣ�
��2��ʵ�鲽������Բ�������������Ӧ����м������������Һ�п����������Ӻ�ͭ���Ӵ��ڣ�
��3��Ϊ��ϴ�ӳ�ȥ������渽�ŵ���������ʣ�ʵ�鲽�������������ˮϴ�Ӿ��壬ԭ�����ñ�ˮϴ�ӿɽ���ϴ�ӹ�����FeSO4•7H2O����ģ�
������̽���̷���FeSO4•7H2O���ȷֽ�IJ��
��֪SO3���۵���16.8�棬�е���44.8�棬��С�������ͼ��ʾ��ʵ��װ�ã�ͼ�м��ȡ��г������Ⱦ�ʡ�ԣ���
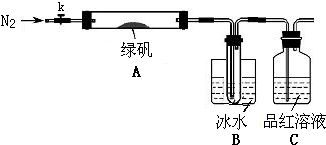
[ʵ�����]
���������Ӻ��װ��A��B�����ԣ�
��ȡһ�����̷���������A�У�ͨ��N2������װ���ڵĿ������ر�k���þƾ��Ƽ���Ӳ�ʲ����ܣ�
�۹۲쵽A �й��������ɫ��B���Թ��ռ�����ɫҺ�壬C����Һ��ɫ��
�ܴ�A�з�Ӧ��ȫ����ȴ�����º�ȡ������Ӧ��������Թ��У����������ܽ⣬ȡ�������뼸��KSCN��Һ����Һ���ɫ��
����Bװ�õ��Թ��е��뼸��BaCl2��Һ����Һ����ǣ�
��4��ʵ��������
����1��B���ռ�����Һ����H2SO4��Һ��
����2��C����Һ��ɫ������֪��������SO2��
����3���ۺϷ�������ʵ��ۺܿ͢���֪�������һ����Fe2O3��
[ʵ�鷴˼]
��5����ָ����С����Ƶ�ʵ��װ�õ����Բ��㣺��Cװ�ú�����һ��β������װ�ã�
��6���ֽ��Ĺ����п��ܺ�������FeO��ȡ����ʵ����������ܽ�����Һ�������Թ��У�ѡ��һ���Լ����𣬸��Լ�����ʵ���b��
a����ˮ��KSCN��Һ b������KMnO4��Һ c��H2O2 d��NaOH��Һ��
���� ��1��̼���Ƶ�ˮ��Һ�Լ��ԣ����۵���Ҫ�ɷ�Ϊ��֬���ڼ�������������ˮ�⣬ˮ�����������ˮ�����ʣ����Գ�ȥ��м��������ۣ�
��2������м�к���������ͭ�������������ʣ�
��3�������ܽ�ȣ�������ʧ��
��4�����ᱵ�ǰ�ɫ����������������ʹƷ����ɫ��
��5�����������Դ��������Ⱦ��
��6��+2����������ʹ����KMnO4��Һ��ɫ��
��� �⣺��1�����۵���Ҫ�ɷ�Ϊ��֬���ڼ�������������ˮ�⣬ˮ�����������ˮ�ĸ�֬�����ƺͱ�����������м������������̼���ƶ�����Ӧ��Ҳ������ˮ��̼���Ƶ�ˮ��Һ���Գ�ȥ��м��������ۣ�
�ʴ�Ϊ�������ۣ�
��2������м�к���������ͭ�������������ʣ������ᷴӦ����Һ�л���������Ӻ�ͭ���ӣ�����мӦ������
�ʴ�Ϊ��Ӧ����м������������Һ�п����������Ӻ�ͭ���Ӵ��ڣ�
��3���̷�������ˮ�����¿��Խ������ܽ�ȣ�������ʧ��
�ʴ�Ϊ���ñ�ˮϴ�ӿɽ���ϴ�ӹ�����FeSO4•7H2O����ģ�
��4����Bװ�õ��Թ��е��뼸��BaCl2��Һ����Һ����ǣ�˵��B���ռ�����Һ����������Һ��Ʒ����Һ��ɫ��˵����SO2���ɣ�
�ʴ�Ϊ��H2SO4��Һ��SO2��
��5�����������Դ��������Ⱦ����Ӧ��β������װ�ã�
�ʴ�Ϊ����Cװ�ú�����һ��β������װ�ã�
��6��+2�������Ӿ��л�ԭ�ԣ���ʹ����KMnO4��Һ��ɫ����ӦΪ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��
�ʴ�Ϊ��b��
���� ������һ��ʵ��̽���⣬�ܽϺõĿ���ѧ�������ͽ����������������ʱҪ��������ṩ��Ϣ��������֪ʶϸ�ķ��������Ŀ�Ѷ��еȣ�

 ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д� �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�| A�� | ϡ�ͺ���ҺpH���� | B�� | ͨ������HCl��c��NH4+������c��Cl-������ | ||
| C�� | c��NH4+��+c��OH-����c��Cl-��+c��H+�� | D�� | c��Cl-����c��H+����c��NH4+�� |
| A�� | $\frac{4n-1}{2}$ | B�� | $\frac{n-m}{3}$ | C�� | $\frac{3m+n}{3}$ | D�� | 3��n-m�� |
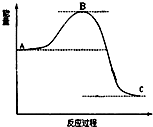 ��֪2SO2��g��ʮO2��g��$?_{����}^{����}$2SO3��g����Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
��֪2SO2��g��ʮO2��g��$?_{����}^{����}$2SO3��g����Ӧ���̵������仯��ͼ��ʾ����ش��������⣺ ��Ҫ��ش��������⣺
��Ҫ��ش��������⣺ �������仯�����ڿ��м������о�������Ҫ��Ӧ�ã�
�������仯�����ڿ��м������о�������Ҫ��Ӧ�ã�