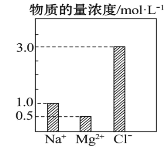
��Ŀ����
����Ŀ��ʵ�����ù����ռ�����0.1mol/L��NaOH��Һ480mL����ش�
(1)������ҪNaOH��������______g��
(2)���������������ձ���ҩ�� ��250mL����ƿ��500mL����ƿ�ݲ�������������ƽ����Ͳ������ʱ������ʹ�õIJ�������______������ţ�����ȱ�ٵ�������______��
(3)ʹ������ƿǰ������е�һ��������______��
(4)������Һʱ���ڼ��㡢�������ܽ⡢��ȴ�������¼������裬����ȷ�IJ���˳��Ϊ______������ţ���
����ҡ�ȣ���ϴ�ӣ��۶��ݣ��ܵߵ�ҡ�ȣ���ת��
(5)���ƹ����У����в�����������ƫ�ߵ���______������ţ���
��δϴ���ձ�����������
�ڳ���NaOH��ʱ��̫����
�۶���ʱ���ӿ̶ȣ�
������ƿ�����������������ˮ��
��NaOH��Һδ��ȴ�����¾�ת�Ƶ�����ƿ��
(6)ijͬѧ��Ũ�������Ƶ�ϡ����Ũ��ƫ�ͣ�����ܵ�ԭ����______(�����)��
������Ͳ��ȡŨ����ʱ�����ӿ̶���
������ƿ������ˮϴ�Ӻ�δ������
��ϴ���ձ��ڱں�ϴ��Һ��ȥ
��ת����Һʱ��������������Һ����
�ݶ���ʱ����������ƿ�̶���
���ݡ�ҡ�Ⱥ�����Һ�İ�Һ����ڿ̶���
���𰸡�2.0 �٢ܢ� ��ͷ�ι� ����Ƿ�©ˮ �ݢڢ٢ۢ� �ۢ� �ۢ�
��������
(1)����m=cVM�������ʵ�������
(2)���ݲ�������ѡȡʵ��������
(3)����ƿʹ��ǰ�ü���Ƿ�©ˮ��
(4)��������һ�����ʵ���Ũ����Һһ�㲽����
(5)�����������������ʵ����ʵ�������Һ�����Ӱ�죬����c=![]() ������������
������������
(6)����c=![]() ������������
������������
(1)����0.1mol/L��NaOH��Һ480mL��Ӧѡ��500mL����ƿ����Ҫ���ʵ�����m(NaOH)=0.1mol/L��0.5L��40g/mol=2.0g��
(2)���Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ�(������Ͳ��ȡˮ)����ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2��3�Σ���ϴ��Һ��������ƿ����ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ��ʱ���ʹ�õIJ������������ձ� ��500mL����ƿ �ݲ��������ʺ�������Ǣ٢ܢݣ�����Ҫ�IJ������������ǣ���ͷ�ιܡ�
(3)����ƿʹ��ǰ�ü���Ƿ�©ˮ��
(4)����һ�����ʵ���Ũ����Һ�IJ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����������ȷ��˳��Ϊ���ݢڢ٢ۢܣ�
(5)��δϴ���ձ������������������ʵ����ʵ���ƫС��ʹ��Һ��Ũ��ƫ�ͣ��ٲ��������⣻
�ڳ���NaOH��ʱ��̫�������³�ȡ���������Ƶ����ʵ���ƫ�ͣ�ʹ��ҺŨ��ƫ�ͣ��ڲ��������⣻
�۶���ʱ���ӿ̶ȣ�������Һ���ƫС��ʹ��ҺŨ��ƫ�۷������⣻
������ƿ�����������������ˮ�������ʵ����ʵ�������Һ�������Ӱ�죬��ҺŨ�Ȳ��䣬�ܲ��������⣻
��NaOH��Һδ��ȴ�����¾�ת�Ƶ�����ƿ����ȴ����Һ�����С��������ҺŨ��ƫ�ݷ������⣻
�ʺ���ѡ���Ǣۢݣ�
(6)������Ͳ��ȡŨ����ʱ�����ӿ̶��ߣ�������ȡ��Ũ����ƫ�࣬���ʵ����ʵ���ƫ��ʹ��Һ��Ũ��ƫ�ߣ��ٲ��������⣻
������ƿ������ˮϴ�Ӻ�δ���������Һ��Ũ�Ȳ������Ӱ�죬�ڲ��������⣻
��ϴ���ձ��ڱں�ϴ��Һ��ȥ���������ʵ����ʵ���ƫС��ʹ��Һ��Ũ��ƫ�ͣ��۷������⣻
��ת����Һʱ��������������Һ�������������ʵ����ʵ���ƫС��ʹ��Һ��Ũ��ƫ�ͣ��ܷ������⣻
�ݶ���ʱ����������ƿ�̶��ߣ�������Һ�����ƫС����Һ��Ũ��ƫ�ߣ��ݲ��������⣻
���ݡ�ҡ�Ⱥ�����Һ�İ�Һ����ڿ̶��ߣ��ò�������������������Һ��Ũ����Ӱ�죬���������⣻
�ʺ���ѡ���Ǣۢܡ�

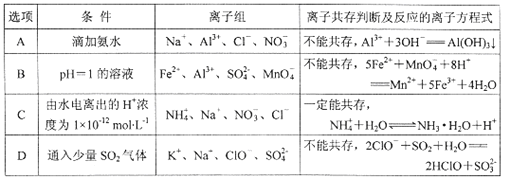
����Ŀ���±���ʾʵ�飬����ͽ��۾���ȷ����
ѡ�� | ʵ�� | ���� | ���� |
A | ��Ũ�Ⱦ�Ϊ0.lmol/LNaCl��NaI�����Һ�еμ�����AgNO3��Һ | ���ֻ�ɫ���� | Ksp(AgCl)>Ksp(AgI) |
B | �����£��ⶨ�����ʵ���Ũ�ȵ� Na2CO3��Na2SO3��Һ��pHֵ | ǰ�ߵ�pHֵ�Ⱥ��ߵĴ� | �ǽ����ԣ�S>C |
C | ��ij��Һ�м��������ữ���Ȼ�����Һ | ��Һ���а�ɫ�������� | ����Һ�к���SO42- |
D | ��FeCl3��KSCN�����Һ�У���������KC1���� | ��Һ��ɫ��dz | FeCl3+3KSCN |
A. A B. B C. C D. D