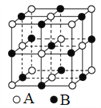
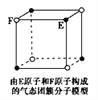
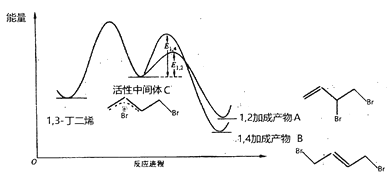
��Ŀ����
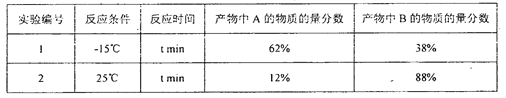
����Ŀ����ͼ��ʾ��ͼ���ʾ����ϸ����Ԫ�ء������a��b��c��d������ͬ��С�������ʣ�A��B��C������ͬ�Ĵ�������ʣ�������ش��������⣺
��1������a��_________���ڶ���ϸ���ڣ�������A�����������������_________��������A�ڶ��ֲ��ϸ���о��ɺ��У�������Ϊϸ����������Ĵ������ʣ���������������������С����A��______________��
��2������b��____________����ij��B������n��b���ӣ�ƽ����Է�������Ϊm����ɵ�2������ɣ����B���ӵ���Է���������ԼΪ____________��
��3������c������ϸ���й���_______�֣�������___________�IJ�ͬ������c�����ͬ��
��4������d��____________��d��__________��ά����D�����ڹ̴������ʡ�
���𰸡������� ��ԭ ֬�� ������ mn��18��n��2�� 4 ������� ���Լ��� ���̴�
��������
�Ķ���ɺ���ͼ��֪�������֪ʶ��������ķ��ࡢ�ֲ����ܣ�֬�ʵķ�����ܣ������ʵĻ�����ɵ�λ���ṹ���ܣ�����ķ��ࡢ�ֲ��ṹ���ܣ��ȸ�����ͼ�������֪ʶ�㣬Ȼ���������Ϣ���н��ͼ��A�ǵ��ۣ�a�������ǣ�B�ǵ����ʣ�b�ǰ����ᣬC��RNA��c�Ǻ��Ǻ����ᣬd���Լ��ء�
��1��ֲ��ϸ���Ĵ���Ӵ��������ǵ��ۻ�����ɵ�λ�������ǣ�����ϸ���д��������Ķ�������ԭ��֬���Ƕ�ֲ��ϸ�������е����ʣ���ͬ������֬�����е��������ڶ��ǣ��Ƕ�ֲ��ϸ�����е����õĴ������ʡ�
��2��b�ǵ����ʵĻ�����ɵ�λ�����ᣬ����������Է�������=�����������������ƽ����������-ˮ�����������������-����������ˮ����Է�����������B���ӵ���Է���������ԼΪmn��18��n��2����
��3��c�Ǻ��Ǻ����ᣬ���Ǻ����Ậ�еļ����A��U��C��G�����ݺ�������IJ�ͬ�γ�4�ֺ��Ǻ����ᡣ
��4����d�Ĺ��ܿ��Կ�����d�����Լ��أ��̴������ʰ����Լ��ء�ά����D�͵��̴���

 �Ķ��쳵ϵ�д�
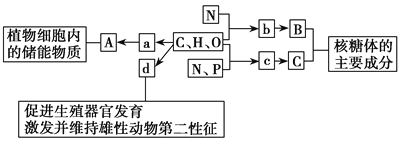
�Ķ��쳵ϵ�д�����Ŀ���������ʵķ�����ȷ���ǣ� ��
�� | �� | �� | ���������� | ���������� | |
A | Na2CO3 | H2SO4 | Cu2��OH��2CO3 | Fe2O3 | SO3 |
B | NaOH | HCl | NaCl | Na2O | NO2 |
C | NaOH | NaHSO4 | CaF2 | MgO | SO2 |
D | KOH | HNO3 | NaHCO3 | CaO | CO2 |
A.AB.BC.CD.D
����Ŀ���췯��(Na2Cr2O7��2H2O)�㷺����ǿ����������������Ը���ʯ(��Ҫ�ɷ�ΪCr2O3,������FeO��Al2O3��SiO2������)Ϊԭ����ȡ�췯�Ƶ���������:

��֪:��CrO42-��Cr2O72-��������ƽ��:2CrO42-+2H+![]() Cr2O72-+H2O����pH<3ʱ����Cr2O72-Ϊ������pH>9ʱ����CrO42-Ϊ����
Cr2O72-+H2O����pH<3ʱ����Cr2O72-Ϊ������pH>9ʱ����CrO42-Ϊ����
�������ڲ�ͬ�¶��µ��ܽ��(g):
�¶�(��) | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
Na2SO4 | 9.0 | 19.4 | 40.8 | 48.8 | 46.7 | 45.3 | 44.1 | 43.7 |
Na2Cr2O7 | 170.2 | 180.1 | 196.7 | 220.5 | 248.4 | 283.1 | 323.8 | 385.4 |
ע32.38�����ϣ��뱥����Һƽ��Ĺ���Ϊ��ˮNa2SO4,������ΪNa2SO4��10H2O��
��Cr3+��ȫ����ʱpHΪ6.8��Cr(OH)3��ʼ�ܽ�ʱpHΪ12��
��ش���������:
(1)���ո���ʯʱ������Na2CrO4�Ļ�ѧ��Ӧ����ʽΪ_________
(2)������ijɷ���__________(�ѧʽ)��
(3)������Ϊ���������С���ϡ�����pH����Ϊ��ͨ�����CO2�����������pHͬ�����Դﵽʵ��Ч����������___________��
(4)��췯����Һ�м�������KCl,����Ũ������ȴ�ᾧ�����˵õ�K2Cr2O7���塣��ȴ��________
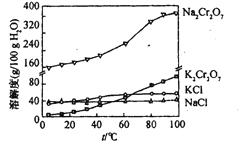
A.80 B.60 C.40 D.10
(5)�ù������ø���Ʒ��ҪΪ��ˮ�����Ʋ����������ظ����ƣ�����ƴӸ���Ʒ���â��(Na2SO4��10H2O)��ʵ�鷽��:���ø���Ʒ����Һ������100:230������ˮ�������Թ�����Na2SO3��Һ������,____�����ˣ�ϴ�ӣ����¸��(ʵ������ʹ�õ��Լ�:ϡH2SO4��NaOH��Һ)��