��Ŀ����
�黯�����ڵ������뵼�壬����ֱ�ӽ�����ת��Ϊ���ܣ��黯�ص�����������ͨ���ݵ�100����������ֻ����10%���ƹ��黯�صȷ�������ܣ�LED���������ǽ��ܼ��ŵ���Ч�ٴ룬��֪�黯�صľ����ṹ����ͼ���Իش���������
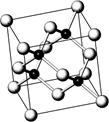
��1������˵����ȷ���� �� ��ѡ����ţ���
A���黯�ؾ����ṹ��NaCl��ͬ B����һ�����ܣ�As��Ga
C���縺��:As��Ga D������ض�����p��Ԫ��
E���뵼��GaP��SiC���黯��Ϊ�ȵ�����
��2���黯���ǽ�(CH3)3Ga��AsH3��MOCVD�����Ʊ��õ��� �÷�Ӧ��700����У���Ӧ�ķ���ʽΪ�� �� ��
AsH3�ռ���״Ϊ�� �� (CH3)3Ga����ԭ���ӻ���ʽΪ�� �� ��
��3��Ga�ĺ�������Ų�ʽΪ�� �� ��
��4��AsH3�е��NH3�ͣ���ԭ���ǣ� �� ��

��1������˵����ȷ���� �� ��ѡ����ţ���
A���黯�ؾ����ṹ��NaCl��ͬ B����һ�����ܣ�As��Ga
C���縺��:As��Ga D������ض�����p��Ԫ��
E���뵼��GaP��SiC���黯��Ϊ�ȵ�����
��2���黯���ǽ�(CH3)3Ga��AsH3��MOCVD�����Ʊ��õ��� �÷�Ӧ��700����У���Ӧ�ķ���ʽΪ�� �� ��
AsH3�ռ���״Ϊ�� �� (CH3)3Ga����ԭ���ӻ���ʽΪ�� �� ��
��3��Ga�ĺ�������Ų�ʽΪ�� �� ��
��4��AsH3�е��NH3�ͣ���ԭ���ǣ� �� ��
��1��BCDE
��2��(CH3)3Ga+AsH3
 GaAs+3CH4
GaAs+3CH4(��
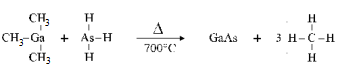 )��������sp2
)��������sp2��3��1s22s22p63s23p63d104s24p1
��4��NH3���Ӽ����γ��������As�縺��С���뾶���Ӽ䲻���γ������
����ֻ�ǰ�ѡ��3������֪ʶ������С���1����Ҫ��ʶ���黯�ؾ����ṹ��NaCl�ļ�������ͬ������ѡ������жϣ���2������ʽ���Կ���ȡ����û�л��ϼ۱仯����3��Ϊ�����ԣ���4��������������

��ϰ��ϵ�д�
�����Ŀ
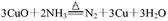
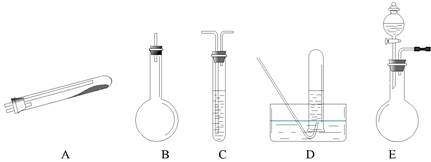
 �����Ҫ������������ �ģ���������ĸ����ͬ����Ϊ��������װ�á�Ҫ��ȡ���ռ�������N2��������������ˮ����������Ӧʹ�õ����������е�
�����Ҫ������������ �ģ���������ĸ����ͬ����Ϊ��������װ�á�Ҫ��ȡ���ռ�������N2��������������ˮ����������Ӧʹ�õ����������е�