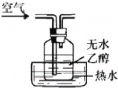
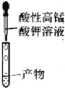
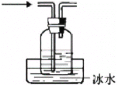
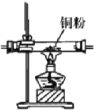
ĢāÄæÄŚČŻ
”¾ĢāÄæ”æO2ŗĶO3ŹĒŃõŌŖĖŲµÄĮ½ÖÖµ„ÖŹ£¬øł¾ŻĘä»ÆѧŹ½Ķź³ÉĻĀĮŠø÷Ģā£ŗ
(1)µČÖŹĮæµÄO2ŗĶO3Ō×ÓøöŹż±ČĪŖ_______________”£µČĪĀµČŃ¹ĻĀ£¬µČĢå»żµÄO2ŗĶO3ĖłµÄÖŹĮæ±ČĪŖ___________”£
(2)ÉčNAĪŖ°¢·ü¼ÓµĀĀŽ³£ŹżµÄŹżÖµ£¬Čē¹ūagŃõĘųÖŠŗ¬ÓŠµÄ·Ö×ÓŹżĪŖb£¬ŌņcgŃõĘųŌŚ±ź×¼×“æöĻĀµÄĢå»żŌ¼ŹĒ_____________L(ÓĆŗ¬NAµÄŹ½×Ó±ķŹ¾)”£
(3)ŅŃÖŖCO”¢O2Į½ÖÖĘųĢåµÄ»ģŗĶĘųĢåµÄĆܶČŌŚĻąĶ¬Ģõ¼žĻĀŹĒĒāĘųµÄ15±¶£¬ŌņøĆ»ģŗĶĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæĪŖ_____”£
(4)ŹµŃéŹŅŠčŅŖÅäÖĘ920mL1mol”¤L-1µÄĻ”H2SO4ČÜŅŗ£¬ŠčŅŖÓĆĮæĶ²ĮæČ”ÅØH2SO4(ĆܶČĪŖ1.84g”¤mL-1£¬ÖŹĮæ·ÖŹżĪŖ98%)µÄĢå»żĪŖ____mL”£
”¾“š°ø”æ1£ŗ1 2£ŗ3 ![]() 30 54.3
30 54.3
”¾½āĪö”æ
øł¾ŻO2ŗĶO3½į¹¹×é³É£¬ÓÉn=![]() ¼ĘĖćO2ŗĶO3µÄŌ×ÓøöŹżŗĶµČĪĀµČŃ¹µČĢå»żµÄO2ŗĶO3µÄÖŹĮæ±Č£»ÓÉ°¢·ü¼ÓµĀĀŽ¶ØĀɼĘĖćŃõĘųµÄĢå»żŗĶ»ģŗĶĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮ棻ŅĄ¾Żc=
¼ĘĖćO2ŗĶO3µÄŌ×ÓøöŹżŗĶµČĪĀµČŃ¹µČĢå»żµÄO2ŗĶO3µÄÖŹĮæ±Č£»ÓÉ°¢·ü¼ÓµĀĀŽ¶ØĀɼĘĖćŃõĘųµÄĢå»żŗĶ»ģŗĶĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮ棻ŅĄ¾Żc= ![]() £¬¼ĘĖćÅØĮņĖįĪļÖŹµÄĮæÅØ¶Č£¬ŅĄ¾ŻČÜŅŗĻ”ŹĶ¹ż³ĢÖŠČÜÖŹµÄĪļÖŹµÄĮæ²»±ä¼ĘĖćŠčŅŖÅØĮņĖįĢå»ż”£
£¬¼ĘĖćÅØĮņĖįĪļÖŹµÄĮæÅØ¶Č£¬ŅĄ¾ŻČÜŅŗĻ”ŹĶ¹ż³ĢÖŠČÜÖŹµÄĪļÖŹµÄĮæ²»±ä¼ĘĖćŠčŅŖÅØĮņĖįĢå»ż”£
(1)O2ŗĶO3¾łÓÉŃõŌ×Ó¹¹³É£¬¶žÕßÖŹĮæĻąµČ£¬Ōņŗ¬Ō×ÓøöŹżĻąµČ£¬¼“ŗ¬ÓŠŌ×ÓŹżÄæÖ®±ČĪŖ1£ŗ1£»µČĪĀµČŃ¹ĻĀ£¬µČĢå»żµÄO2ŗĶO3£¬¼“ĪļÖŹµÄĮæĻąµČ£¬øł¾Żn=![]() £¬ĶĘ³öm=nMæÉÖŖ¶žÕßÖŹĮæÖ®±Čm(O2)£ŗm(O3)=32g/mol£ŗ48g/mol=2£ŗ3£»“š°øĪŖ1£ŗ1£¬2£ŗ3”£
£¬ĶĘ³öm=nMæÉÖŖ¶žÕßÖŹĮæÖ®±Čm(O2)£ŗm(O3)=32g/mol£ŗ48g/mol=2£ŗ3£»“š°øĪŖ1£ŗ1£¬2£ŗ3”£
(2)ÓÉagŃõĘųÖŠŗ¬ÓŠµÄ·Ö×ÓŹżĪŖb£¬ŌņcgŃõĘųŗ¬ÓŠ·Ö×ÓŹżÄæĪŖN=b”Į![]() £¬ŃõĘųĪļÖŹµÄĮæĪŖn=
£¬ŃõĘųĪļÖŹµÄĮæĪŖn=  =
= ![]() mol£¬Ōņ±źæöĻĀŃõĘųĢå»żĪŖV(O2)=
mol£¬Ōņ±źæöĻĀŃõĘųĢå»żĪŖV(O2)=![]() mol”Į22.4L/mol=
mol”Į22.4L/mol=![]() L£»“š°øĪŖ
L£»“š°øĪŖ![]() ”£
ӣ
(3)øł¾Ż°¢·ü¼ÓµĀĀŽ¶ØĀÉÖŖ£ŗ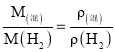 £¬ÓÉCO”¢O2Į½ÖÖĘųĢåµÄ»ģŗĶĘųĢåµÄĆܶČŌŚĻąĶ¬Ģõ¼žĻĀŹĒĒāĘųµÄ15±¶£¬¼“
£¬ÓÉCO”¢O2Į½ÖÖĘųĢåµÄ»ģŗĶĘųĢåµÄĆܶČŌŚĻąĶ¬Ģõ¼žĻĀŹĒĒāĘųµÄ15±¶£¬¼“![]() =15£¬Ņņ¶ųM(»ģ) =2”Į15=30£»“š°øĪŖ30”£
=15£¬Ņņ¶ųM(»ģ) =2”Į15=30£»“š°øĪŖ30”£
(4)ÅäÖĘ920mL1mol”¤L-1µÄĻ”H2SO4ČÜŅŗ£¬ŅņĪŖŹµŃéŹŅƻӊ920mL¹ęøńµÄČŻĮæĘ棬ĖłŅŌӦєȔ1000mL¹ęøńµÄČŻĮæĘ棬ÓÉc=![]() £¬µĆ³öc(ÅØH2SO4)=
£¬µĆ³öc(ÅØH2SO4)=![]() =18.4mol/L£¬øł¾ŻĻ”ŹĶĒ°ŗóČÜÖŹµÄĪļÖŹµÄĮæ²»±ä£¬Ōņ1000mL”Į10-3”Į1mol/L=V”Į10-3L”Į18.4mol/L£¬½āµĆV=54.3mL£»“š°øĪŖ54.3”£
=18.4mol/L£¬øł¾ŻĻ”ŹĶĒ°ŗóČÜÖŹµÄĪļÖŹµÄĮæ²»±ä£¬Ōņ1000mL”Į10-3”Į1mol/L=V”Į10-3L”Į18.4mol/L£¬½āµĆV=54.3mL£»“š°øĪŖ54.3”£

 ĮĮµć¼¤»ī¾«±ąĢįÓÅ100·Ö“óŹŌ¾ķĻµĮŠ“š°ø
ĮĮµć¼¤»ī¾«±ąĢįÓÅ100·Ö“óŹŌ¾ķĻµĮŠ“š°ø ÖĒȤŗ®¼Ł×÷ŅµŌĘÄĻæĘ¼¼³ö°ęÉēĻµĮŠ“š°ø
ÖĒȤŗ®¼Ł×÷ŅµŌĘÄĻæĘ¼¼³ö°ęÉēĻµĮŠ“š°ø”¾ĢāÄæ”æŅŃÖŖ³£ĪĀĻĀÅضČĪŖ0.1mol/LµÄ¼øÖÖČÜŅŗµÄpHČēÓŅĻĀ±ķ”£ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ£Ø £©
ČÜÖŹ | pH |
NaF | 7.5 |
Na2CO3 | 11.6 |
NaClO | 9.7 |
NaHCO3 | 8.3 |
A. Ķ¬ĪĀ¶ČĶ¬ÅضČĻĀ£¬ĖįÓÉĒæµ½ČõµÄĖ³ŠņĪŖ£ŗHF£¾H2CO3£¾HClO
B. Ė®½ā·½³ĢŹ½£ŗF-+H2O![]() HF+OH-µÄĘ½ŗā³£ŹżĪŖ1”Į10-13
HF+OH-µÄĘ½ŗā³£ŹżĪŖ1”Į10-13
C. ½«CO2ĶØČė0.lmol/LNa2CO3ČÜŅŗÖĮČÜŅŗ³ŹÖŠŠŌ£¬ŌņČÜŅŗÖŠ£ŗ2c(CO32-)+c(HCO3-)=0.1mol/L
D. µČĪļÖŹµÄĮæµÄNaFŗĶHF»ģŗĻČÜŅŗÖŠĮ£×ÓÅØ¶Č“óŠ”¹ŲĻµĪŖ£ŗc(HF)>c(Na+)>c(F-)>c (H+)>c(OH-)
”¾ĢāÄæ”æŅ»¶ØĪĀ¶ČĻĀ£¬ŌŚČżøöĢå»ż¾łĪŖ2.0LµÄŗćČŻĆܱÕČŻĘ÷ÖŠ·Ö±š¼ÓČėŅ»¶ØĮæµÄX£¬·¢Éś·“Ó¦£ŗpX(g) ![]() Y(g)+Z(g)£¬Ļą¹ŲŹż¾ŻČēĻĀ±ķĖłŹ¾£ŗ
Y(g)+Z(g)£¬Ļą¹ŲŹż¾ŻČēĻĀ±ķĖłŹ¾£ŗ
ČŻĘ÷±ąŗÅ | ĪĀ¶Č(”ę) | ĘšŹ¼ĪļÖŹµÄĮæ(mol) | Ę½ŗāĪļÖŹµÄĮæ(mol) | |
X(g) | Y(g) | Z(g) | ||
¢ń | 387 | 0.20 | 0.080 | 0.080 |
¢ņ | 387 | 0.40 | 0.160 | 0.160 |
¢ó | T | 0.20 | 0.090 | 0.090 |
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ČōČŻĘ÷¢ńÖŠ·“Ó¦¾10min“ļµ½Ę½ŗā£¬ŌņĒ°10minÄŚYµÄĘ½¾ł·“Ó¦ĖŁĀŹv(Y)=___________”£ČŻĘ÷¢ńŗĶČŻĘ÷¢ņÖŠĘšŹ¼Ź±XµÄ·“Ó¦ĖŁĀŹv(X)¢ń___________v(X)¢ņ(Ģī”°“óÓŚ”±”°Š”ÓŚ”±»ņ”°µČÓŚ”±)”£
£Ø2£©ŅŃÖŖøĆÕż·“Ó¦ĪŖ·ÅČČ·“Ó¦£¬ŌņT___________387(Ģī”°“óÓŚ”±»ņ”°Š”ÓŚ”±)£¬ÅŠ¶ĻĄķÓÉŹĒ___________”£
£Ø3£©·“Ó¦·½³ĢŹ½ÖŠXµÄ»Æѧ¼ĘĮæŹżpµÄȔֵĪŖ___________£¬ČŻĘ÷¢ņÖŠXµÄĘ½ŗā×Ŗ»ÆĀŹĪŖ___________”£ČōĘšŹ¼Ź±ĻņČŻĘ÷¢ńÖŠ³äČė0.1molX”¢0.15molYŗĶ0.10molZ£¬Ōņ·“Ó¦½«Ļņ___________ (Ģī”°Õż”±»ņ”°Äę”±)·“Ó¦·½Ļņ½ųŠŠ£¬ÅŠ¶ĻĄķÓÉŹĒ_____________________________________________________”£
”¾ĢāÄæ”æ(1)0.3 molµÄĘųĢ¬øßÄÜČ¼ĮĻŅŅÅšĶé(B2H6)ŌŚŃõĘųÖŠČ¼ÉÕ£¬Éś³É¹ĢĢ¬µÄČżŃõ»Æ¶žÅšŗĶŅŗĢ¬Ė®£¬·Å³ö649.5 kJČČĮ棬ĘäČČ»Æѧ·½³ĢŹ½ĪŖ____”£
(2)³¬ŅōĖŁ·É»śŌŚĘ½Į÷²ć·ÉŠŠŹ±£¬Ī²ĘųÖŠµÄNO»įĘĘ»µ³ōŃõ²ć”£æĘѧ¼ŅÕżŌŚŃŠ¾æĄūÓĆ“ß»Æ¼¼Źõ½«Ī²ĘųÖŠµÄNOŗĶCO×Ŗ±ä³ÉCO2ŗĶN2£¬»Æѧ·½³ĢŹ½ĪŖ2NO£«2CO![]() 2CO2£«N2£¬ĪŖĮĖ²ā¶ØŌŚÄ³Ö֓߻ƼĮ×÷ÓĆĻĀµÄ·“Ó¦ĖŁĀŹ£¬ŌŚÄ³ĪĀ¶ČĻĀÓĆĘųĢå“«øŠĘ÷²āµĆ²»Ķ¬Ź±¼äµÄNOŗĶCOÅضČČē±ķ£ŗ
2CO2£«N2£¬ĪŖĮĖ²ā¶ØŌŚÄ³Ö֓߻ƼĮ×÷ÓĆĻĀµÄ·“Ó¦ĖŁĀŹ£¬ŌŚÄ³ĪĀ¶ČĻĀÓĆĘųĢå“«øŠĘ÷²āµĆ²»Ķ¬Ź±¼äµÄNOŗĶCOÅضČČē±ķ£ŗ
Ź±¼ä/s | 0 | 1 | 2 |
c(NO)/ mol”¤L£1 | 1.00”Į10£3 | 4.50”Į10£4 | 2.50”Į10£4 |
c(CO)/mol”¤L£1 | 3.60”Į10£3 | 3.05”Į10£3 | 2.85”Į10£3 |
Ź±¼ä/s | 3 | 4 | 5 |
c(NO)/ mol”¤L£1 | 1.50”Į10£4 | 1.00”Į10£4 | 1.00”Į10£4 |
c(CO)/ mol”¤L£1 | 2.75”Į10£3 | 2.70”Į10£3 | 2.70”Į10£3 |
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ŁĒ°2sÄŚµÄĘ½¾ł·“Ó¦ĖŁĀŹ¦Ō(N2)£½____£»
¢ŚÉĻŹöĢõ¼žĻĀ£¬øĆ·“Ó¦µÄĘ½ŗā³£ŹżĪŖ____£»
¢ŪÉĻŹöĢõ¼žĻĀ£¬²āµĆijŹ±æĢ·“Ó¦ĢåĻµÖŠø÷ĪļÖŹµÄĪļÖŹµÄĮæÅØ¶Č¾łĪŖ0.01 mol/L£¬Ōņ“ĖŹ±·“Ó¦“¦ÓŚ____דĢ¬”£(Ģī”°Ę½ŗā”±»ņ”°ĻņÓŅ½ųŠŠ”±»ņ”°Ļņ×ó½ųŠŠ”±)
(3)ŹµŃéŹŅ³£ÓĆ0.10 mol/L KMnO4±ź×¼ĖįŠŌČÜŅŗĄ“²ā¶ØH2C2O4ѳʷµÄ“æ¶Č(±ź×¼ŅŗµĪ“ż²āŅŗ)£¬Ęä·“Ó¦ŌĄķĪŖ£ŗ5H2C2O4£«2MnO4££«6H£«=10CO2”ü£«2Mn2+£«8H2O”£
¢ŁKMnO4±ź×¼ŅŗӦװŌŚ____(Ģī”°ĖįŹ½”±»ņ”°¼īŹ½”±)µĪ¶Ø¹Ü£»
¢Ś ĒåĖ®Ļ“¾»µĪ¶Ø¹ÜŗóÖ±½Ó×°Čė±ź×¼Ņŗ£¬Ōņ²ā¶Ø½į¹ū»į____£»(Ģī”°Ę«“ó”±»ņ”°Ę«Š””±»ņ”°²»±ä”±)
¢Ū µĪ¶Ø¹ż³ĢÖŠ·¢ĻÖŅ»¶ĪŹ±¼äŗó·“Ó¦ĖŁĀŹĆ÷ĻŌ¼Óæģ£¬³żČ„ĪĀ¶ČµÄÓ°Ļģ£¬ÄćČĻĪŖ×īÓŠæÉÄܵÄŌŅņŹĒ____”£
”¾ĢāÄæ”æĻĀĮŠĄė×Ó·½³ĢŹ½µÄŹéŠ“¼°ĘĄ¼Ū£¬¾łŗĻĄķµÄŹĒ
Ń”Ļī | Ąė×Ó·½³ĢŹ½ | ĘĄ¼Ū |
A | ÓĆĶµē¼«µē½ā±„ŗĶKClČÜŅŗ£ŗ2H2O+2Cl- | ÕżČ·£ŗCl-µÄŹ§µē×ÓÄÜĮ¦±ČOH-Ēæ |
B | ĻņCuSO4ČÜŅŗÖŠĶØČė¹żĮæµÄH2SĘųĢå£ŗCu2++H2S=CuS”ż+2H+ | “ķĪó£ŗH2SµÄĖįŠŌ±ČH2SO4Čõ |
C | Ba(HCO3)2ČÜŅŗÓė×ćĮæµÄNaOHČÜŅŗ·“Ó¦£ŗBa2++HCO3- +OH- ØTBaCO3”ż+H2O | “ķĪó£ŗBa2+ÓėHCO3-ĻµŹż±ČÓ¦ĪŖ1:2 |
D | ¹żĮæSO2ĶØČėµ½NaClOČÜŅŗÖŠ£ŗSO2+ClO- +H2O= HClO+HSO3- | ÕżČ·£ŗH2SO3µÄĖįŠŌ±ČHClOĒæ |
A.AB.BC.CD.D
”¾ĢāÄæ”æ±źŗÅĪŖ¢Ł-¢āµÄŌŖĖŲ£¬ŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆČēĻĀ£ŗ
Ö÷×å ÖÜĘŚ | ¢ńA | ¢ņA | ¢óA | ¢ōA | ¢õA | ¢öA | ¢÷A | 0×å |
1 | ¢Ł | ¢Ś | ||||||
2 | ¢Ū | ¢Ü | ¢Ż | ¢Ž | ||||
3 | ¢ß | ¢ą | ¢į | ¢ā |
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¢ŚŗÅŌŖĖŲŹĒ______![]() ĢīŌŖĖŲ·ūŗÅ
ĢīŌŖĖŲ·ūŗÅ![]() £¬¢įŗÅŌŖĖŲµÄĄė×Ó½į¹¹Ź¾ŅāĶ¼ĪŖ______
£¬¢įŗÅŌŖĖŲµÄĄė×Ó½į¹¹Ź¾ŅāĶ¼ĪŖ______
![]() ČĪŠ“Į½ÖÖ
ČĪŠ“Į½ÖÖ![]() ”£
ӣ
£Ø3£©ÓƵē×ÓŹ½±ķŹ¾¢Ł¢ÜŗÅŌŖĖŲŠĪ³ÉµÄ×ī¼ņµ„»ÆŗĻĪļµÄŠĪ³É¹ż³Ģ______
£Ø4£©¢ŪµÄ×īøß¼ŪŃõ»ÆĪļÓė¢ąµÄµ„ÖŹŌŚµćČ¼Ģõ¼žĻĀ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½______
£Ø5£©¢Ł”¢¢Ż”¢¢ßŗÅŌŖĖŲŠĪ³ÉµÄ»ÆŗĻĪļµÄµē×ÓŹ½ŹĒ______£¬øĆ»ÆŗĻĪļĖłŗ¬»Æѧ¼üµÄĄąŠĶĪŖ______