��Ŀ����
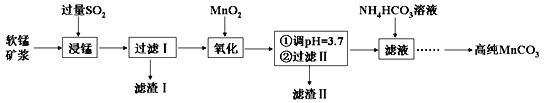
����Ŀ����������Ҫ�Ĺ�ҵԭ�ϣ�������������ϡ�ҩ�ըҩ�����ϡ�ϴ�Ӽ������صȣ�
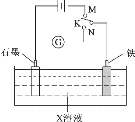
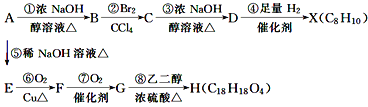
��ijͬѧ��ʵ�����и���2SO2(g)+O2(g)![]() 2SO3(g) ��H =��196.6kJmol-1�������ͼ��ʾʵ��װ�����Ʊ�SO3���壬������������⣺
2SO3(g) ��H =��196.6kJmol-1�������ͼ��ʾʵ��װ�����Ʊ�SO3���壬������������⣺
����Aװ���м���Na2SO3�����ͬʱ������Ӽ���ˮ��Ȼ���ٵμ�Ũ���ᣬ�Ӽ���ˮ��������________��
��װ��D��������________��________��________��
��ʵ�鿪ʼʱ�IJ���˳��Ϊ��_______��
a.�ȼ���E���ľƾ��ƺ������ͨ��E
b.�Ƚ��������ͨ��E�����E���ľƾ���
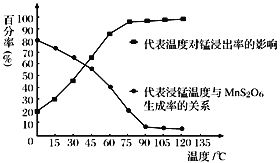
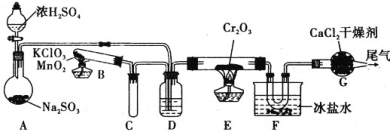
��Ũ�������ǿ�����ԣ�ijͬѧ������װ��������ľ̿�ܷ�Ũ����������CO2
��������������������������װ�õ���ȷ˳����(�ø��ӿ���ĸ��ʾ) ________��
�����ܱ�������CO2��ʵ��������________��
��д��װ�ö��п��ܷ��������ӷ�Ӧ����ʽ________��
���𰸡�ˮ��Ũ�������÷��ȣ�������![]() ���ݳ� ʹ
���ݳ� ʹ![]() ��
��![]() ��Ͼ��ȣ�ͨ���۲����ݿ�����������������ٶ� ��ֹˮ����������
��Ͼ��ȣ�ͨ���۲����ݿ�����������������ٶ� ��ֹˮ����������![]() ��Ӧ
��Ӧ ![]()
![]() ����Ʒ�첻��ɫ�����в�����ɫ����
����Ʒ�첻��ɫ�����в�����ɫ���� ![]()
��������
�Ţ�ʵ����Aװ����ȡSO2���Ӽ���ˮ��������ˮ��Ũ����������SO2�ķų���
������SO2��O2��E�Թ����Դ��������������Ʊ�SO3������SO3����ˮ��Ϊʵ�鰲ȫ����ֹ�����ж��Ա�õ�SO3���壬����E�Թܵ�����Ӧ���Ϊ��ַ�Ӧ��ԭ�����ó�֣���ֹ�˷ѣ�����E�Թܵ�����Ҫ��ֻ�ϣ��������ܹ��죻
��Ϊ�˳�����÷�Ӧ��ȼ���E���ľƾ��ƺ������ͨ��E��
�Ƣٸ���ʵ����Ũ��������ľ̿�ķ�Ӧԭ������������Һ�����·�Ӧ������ѡ����װ�ã�������ؿ��������������ø������������Һ��SO2����ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ�����֤ľ̿�ɱ�Ũ����������CO2����ͨ��������̼��ʹ�����ʯ��ˮ�����ȷ�ϣ�
������Ϊ�����ʯ��ˮ�������ʯ��ˮ�Ͷ�����̼��Ӧ����ǣ������������Ư���ԣ�����������ʹƷ����ɫ����װ���ø������������Һ��SO2����ͨ��Ʒ����Һ����ɫ��֤���ж�����̼���ɣ�
�۶������������Ը��������Һ����������ԭ��Ӧ����д���ӷ���ʽ��
�Ţ�ʵ����Aװ����ȡSO2���Ʊ�ԭ������Ϊ��![]() ����Ӧͨ�����Ƚ��У�����SO2�ݳ������ԼӼ���ˮ��������ˮ��Ũ�������÷��ȣ�������SO2�ķų����ʴ�Ϊ��ˮ��Ũ�������÷��ȣ�������SO2���ݳ���
����Ӧͨ�����Ƚ��У�����SO2�ݳ������ԼӼ���ˮ��������ˮ��Ũ�������÷��ȣ�������SO2�ķų����ʴ�Ϊ��ˮ��Ũ�������÷��ȣ�������SO2���ݳ���
������SO2��O2��E�Թ�����![]() �����������������Ʊ�SO3������SO3����ˮ��Ϊʵ�鰲ȫ����ֹ�����ж��Ա�õ�SO3���壬����E�Թܵ�����Ӧ�������SO2��O2�ڴ������淴Ӧ����SO3��Ϊ��ַ�Ӧ��ԭ�����ó�֣���ֹ�˷ѣ�����E�Թܵ�����Ҫ��ֻ�ϣ��������ܹ��죻���ԣ�װ��D�������Ǣ�ʹSO2��O2��Ͼ��ȣ�ͨ���۲����ݿ�����������������ٶȣ���ֹˮ����������SO3��Ӧ���ʴ�Ϊ��ʹSO2��O2��Ͼ��ȣ�ͨ���۲����ݿ�����������������ٶȣ���ֹˮ����������SO3��Ӧ��
�����������������Ʊ�SO3������SO3����ˮ��Ϊʵ�鰲ȫ����ֹ�����ж��Ա�õ�SO3���壬����E�Թܵ�����Ӧ�������SO2��O2�ڴ������淴Ӧ����SO3��Ϊ��ַ�Ӧ��ԭ�����ó�֣���ֹ�˷ѣ�����E�Թܵ�����Ҫ��ֻ�ϣ��������ܹ��죻���ԣ�װ��D�������Ǣ�ʹSO2��O2��Ͼ��ȣ�ͨ���۲����ݿ�����������������ٶȣ���ֹˮ����������SO3��Ӧ���ʴ�Ϊ��ʹSO2��O2��Ͼ��ȣ�ͨ���۲����ݿ�����������������ٶȣ���ֹˮ����������SO3��Ӧ��
��Ϊ�˳�����÷�Ӧ��ȼ���E���ľƾ��ƺ������ͨ��E���ʴ�Ϊ��a��
�Ƣٸ���ʵ����Ũ��������ľ̿�ķ�Ӧԭ������������Һ�����·�Ӧ������ѡ����װ�ã�������ؿ��������������ø������������Һ��SO2����ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ�����֤ľ̿�ɱ�Ũ����������CO2����ͨ��������̼��ʹ�����ʯ��ˮ�����ȷ�ϣ�����װ�õ���ȷ˳��ΪA��F��E��C��D��B���ʴ�Ϊ��A��F��E��C��D��B��
������Ϊ�����ʯ��ˮ�������ʯ��ˮ�Ͷ�����̼��Ӧ����ǣ������������Ư���ԣ�����������ʹƷ����ɫ����װ���ø������������Һ��SO2����ͨ��Ʒ����Һ����ɫ��֤���ж�����̼���ɣ��ʴ�Ϊ������Ʒ�첻��ɫ�����в�����ɫ������
�۶������������Ը��������Һ��Ӧ���ӷ���ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

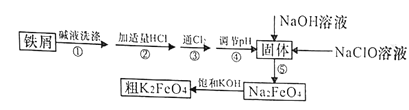
����Ŀ���Է���м(����������)�Ʊ��������(K2FeO4)����������ͼ��ʾ��
��֪��25��ʱ��һЩ�����������↑ʼ��������ȫ����ʱ��pH���±���ʾ��
M(OH)m | PH | |
��ʼ���� | ������ȫ | |
Fe (OH)3 | 2.53 | 2.94 |
Ni(OH)2 | 7.60 | 9.75 |
(1)K2FeO4����Ԫ�صĻ��ϼ�Ϊ________________��
(2)����Һϴ������Ŀ���dz�ȥ��м��������ۣ�ʵ��һ��ѡ��Na2CO3��Һ���ۣ�ѡ��Na2CO3��Һ���۵�ԭ����____________________________(�����ӷ���ʽ��ʾ)��
(3)����۷�����Ӧ�����ӷ���ʽΪ___________________��
(4)������ǽ�Fe(OH)3��������ΪNa2FeO4��ͬʱNaClOת��ΪNaCl��������1mol Na2FeO4����NaClO������Ϊ______g������ܵ���pH�ķ�Χ��_______��
(5)�õζ����ⶨ���ƴ�K2FeO4�Ĵ���(������KI����Ӧ)��ȡ0.220g��K2FeO4��Ʒ���������������ữ��KI��Һ����ַ�Ӧ����0.200mol��L��1Na2S2O3����Һ�ζ����ɵ�I2���ζ����ı���Һ�����Ϊ20.00mL���漰�ķ�Ӧ�У�FeO42����4I����8H����Fe2����2I2��4H2O��2S2O32����I2��S4O62����2I����
�ٵζ�ʱѡ�õ�ָʾ��Ϊ______���ζ��յ������Ϊ_____________��
�ڴ�K2FeO4�Ĵ���Ϊ_____________��