��Ŀ����
��ʵ���֪�õ�ⷨ�����Һ�еĽ������ӻ�ԭΪ��������ʱ���缫��ͨ���ĵ����Q�����ڽ��������ʵ���n�ͽ������ӻ��ϼ�a�ij˻���Q=F��na������F��һ��������Ϊ�����ڳ�������������������أ������ӵ�����NA=6.023��1023 mol-1�����ӵ����e=1.6��10-19 C,Cu��Ħ��������64��10-3 kg��mol-1����
��1������������ڳ���F��������Կ���Ϊ��λ��������λ��Ч���֣���
��2������������ͭ��Һ���1 kg�Ľ���ͭ��ͨ�����۵ĵ�����Ƕ��٣�
��3���õ�Ƶķ����ڰ뾶ΪR��ͭ�������ȵض��Ϻܱ������㣬�ڵ�Ʋ���ͭ��������������������һ�缫��ʲô���ϣ�������ΪI��ͨ��ʱ��Ϊt���������ԭ������ΪA�����������ܶ�Ϊ�ѣ���Ʋ�ĺ��d��
��1������������ڳ���F��������Կ���Ϊ��λ��������λ��Ч���֣���
��2������������ͭ��Һ���1 kg�Ľ���ͭ��ͨ�����۵ĵ�����Ƕ��٣�
��3���õ�Ƶķ����ڰ뾶ΪR��ͭ�������ȵض��Ϻܱ������㣬�ڵ�Ʋ���ͭ��������������������һ�缫��ʲô���ϣ�������ΪI��ͨ��ʱ��Ϊt���������ԭ������ΪA�����������ܶ�Ϊ�ѣ���Ʋ�ĺ��d��
(1)F=9.64��104 C��mol-1
��2��Q=3.0��106 C
��3������������d=
��2��Q=3.0��106 C
��3������������d=

��1���������֪F=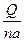 ������na�˻�Ϊת�Ƶĵ��ӵ����ʵ��������Է����ڳ���F�ĺ�����1 mol���ӵĵ��������F=NA��e���������ݿɵ�
������na�˻�Ϊת�Ƶĵ��ӵ����ʵ��������Է����ڳ���F�ĺ�����1 mol���ӵĵ��������F=NA��e���������ݿɵ�
F=NA��e=1.60��10-19C��6.023��1023 mol-1=9.64��104 C��mol-1��
��2��1 kgͭ�����ʵ���n=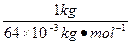 ="15.6" mol��ͭ����a=2,������Q=Fna=9.64��104 C��mol-1��15.6 mol��2=3.0��106 C��
="15.6" mol��ͭ����a=2,������Q=Fna=9.64��104 C��mol-1��15.6 mol��2=3.0��106 C��
��3���ɵ��ԭ����֪�����ƽ������������Ʋ������������ȷ��ͭ�������������������������ɵ�ⶨ�ɿɵó�ͨ���ĵ����Q=Fna������a=1,�����������ʵ���n=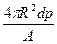 ��
��
�����֪���d= ��
��
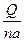 ������na�˻�Ϊת�Ƶĵ��ӵ����ʵ��������Է����ڳ���F�ĺ�����1 mol���ӵĵ��������F=NA��e���������ݿɵ�
������na�˻�Ϊת�Ƶĵ��ӵ����ʵ��������Է����ڳ���F�ĺ�����1 mol���ӵĵ��������F=NA��e���������ݿɵ�F=NA��e=1.60��10-19C��6.023��1023 mol-1=9.64��104 C��mol-1��
��2��1 kgͭ�����ʵ���n=
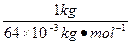 ="15.6" mol��ͭ����a=2,������Q=Fna=9.64��104 C��mol-1��15.6 mol��2=3.0��106 C��
="15.6" mol��ͭ����a=2,������Q=Fna=9.64��104 C��mol-1��15.6 mol��2=3.0��106 C����3���ɵ��ԭ����֪�����ƽ������������Ʋ������������ȷ��ͭ�������������������������ɵ�ⶨ�ɿɵó�ͨ���ĵ����Q=Fna������a=1,�����������ʵ���n=
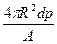 ��
�������֪���d=
 ��
��
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 2NH3(g)+ O2(g)���ش��������⣺
2NH3(g)+ O2(g)���ش��������⣺
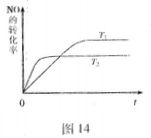
 2NO��g�����ǵ�������β���к���NO��ԭ��֮һ��T��ʱ�����ݻ�Ϊ2 L���ܱ������г���10molN2��5molO2���ﵽƽ���NO�����ʵ���Ϊ2mol����T��ʱ�÷�Ӧ��ƽ�ⳣ��K= ��������������С�������λ���֣�
2NO��g�����ǵ�������β���к���NO��ԭ��֮һ��T��ʱ�����ݻ�Ϊ2 L���ܱ������г���10molN2��5molO2���ﵽƽ���NO�����ʵ���Ϊ2mol����T��ʱ�÷�Ӧ��ƽ�ⳣ��K= ��������������С�������λ���֣�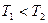 ��
��