��Ŀ����
10�� �ݱ�����ijЩ�����һ���������Ե�뱣�${\;}_{86}^{222}$Rn�����Ӷ�����������˺�����ش�
�ݱ�����ijЩ�����һ���������Ե�뱣�${\;}_{86}^{222}$Rn�����Ӷ�����������˺�����ش���1����ԭ�ӵ���������222����������86����������136��
��2���뽫��ͼRn��ԭ�ӽṹʾ��ͼ��ȫ��
��3�������Rn��ԭ�ӽṹԤ������Ļ�ѧ���ʣ�
A���dz����ã������������ȷǽ������ʷ�Ӧ
B���Ƚϻ��ã������ƵȽ�����Ӧ
C����̫���ã��뵪����������
D���������������ʷ�����Ӧ
��ѡ��Dѡ�������Rnԭ�������������Ѵﵽ8�����ȶ��ṹ��������ȶ���
��4���о����֣�����˥��Ϊ${\;}_{86}^{222}$Rn���ʳ�${\;}_{86}^{222}$RnΪ������������˥��Ϊ${\;}_{86}^{220}$Rn���ʽ�${\;}_{86}^{220}$Rn��Ϊ�����������˥��Ϊ${\;}_{86}^{219}$Rn���ʽ�${\;}_{86}^{219}$Rn��Ϊ�������${\;}_{86}^{222}$Rn��${\;}_{86}^{220}$Rn��${\;}_{86}^{219}$Rn��AB������ĸ��ţ���
A��ͬ��Ԫ�� B��ͬλ�� C��ͬ�ֺ��� D��ͬ��ԭ�ӣ�
���� ��1��ԭ�ӷ���ZA X��X��ʾԪ�ط��ţ�Z������������A������������A����������=Z����������+N�����������ݴ˽�ɣ�
��2��뱴���0�壬�������ڣ�������Ϊ86�����ڲ������Ϊ2������������Ϊ8������������Ų�18�����ӣ��ݴ˵ó����ɣ�
��3���Ԫ�ص������������ﵽ��8�����ӵ��ȶ��ṹ�����ȶ��������ã������������ʷ�Ӧ���ݴ�ѡ�ɣ�
��4����������ͬ�IJ�ͬԭ������ͬ��Ԫ�صIJ�ͬ���أ��ݴ˽�ɣ�
��� �⣺��1��86222Rn��������Ϊ86��������Ϊ222��������N=A-Z=222-86=136���ʴ�Ϊ��222��86��136��
��2��뱴���0�壬�������ڣ�������Ϊ86�����ڲ������Ϊ2������������Ϊ8������������Ų�18�����ӣ�ӦΪ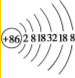 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
����3�������Ԫ�ص������������ﵽ��8�����ӵ��ȶ��ṹ����������������Ӧ����A����Ҳ�����Ʒ�Ӧ����B����뱲����ã���������������N�Ų���ͬ�����뵪�����ʲ�ͬ����C����뱺ܲ����ã������������ʷ�Ӧ����D��ȷ����ѡD��
�ʴ�Ϊ��D����Rnԭ�������������Ѵﵽ8�����ȶ��ṹ��������ȶ���
��4��${\;}_{86}^{222}$Rn��${\;}_{86}^{220}$Rn��${\;}_{86}^{219}$Rn����������ͬ����Ϊ86��������ͬ��Ԫ�أ�������������ͬ�������ڲ�ͬ��ԭ�ӣ���ѡ��AB��
���� ������Ҫ������Ǻ��صĸ��ԭ�ӽṹʾ��ͼ������ĺ��壬�ѶȽϴ�

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д� ��ͬ���칹���У������ϵ�һ�ȴ���ֻ��һ�ֵĽṹ�У������������칹����������
��ͬ���칹���У������ϵ�һ�ȴ���ֻ��һ�ֵĽṹ�У������������칹����������| A�� | 7�� | B�� | 6�� | C�� | 5�� | D�� | 4�� |
| A�� | 32 | B�� | 67 | C�� | 99 | D�� | 166 |
| A�� | ����NH4Cl��Һ�У�c��H+��+c��Cl-��=c��NH4+��+2c��NH3•H2O��+c��OH-�� | |
| B�� | ��AlCl3��Һ��Na2SO3��Һ�ֱ����ɲ����գ��õ�Al2O3��Na2SO3 | |
| C�� | 25�棬��10mLpH=a��������100mLpH=b ��Ba��OH��2��Һ���ǡ���кͣ���a+b=14 | |
| D�� | 25�棬Ka ��HF��=3.6��10-4��Ka��CH3COOH��=1.75��10-5��0.1mol/L ��NaF��Һ��0.1mol/L ��CH3COOK��Һ��ȣ�c��Na+��-c��F-����c��K+��-c��CH3COO-�� |
| A�� | ±�ص��ʵ���������������7 | |
| B�� | ���ϵ��£�±��ԭ�ӵĵ��Ӳ����������࣬�뾶���μ�С | |
| C�� | ±�ص�����H2���ϵ����׳̶�ΪF2��Cl2��Br2��I2 | |
| D�� | ��F��I��ԭ�Ӻ˶��������ӵ������������μ�����ԭ�ӵõ����������μ�����Ԫ�صķǽ��������μ��� |
��
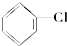 ��
��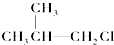 �ۣ�CH3��3C-CH2Cl��CHCl2-CHBr2��
�ۣ�CH3��3C-CH2Cl��CHCl2-CHBr2��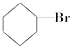 ��CH3Cl��
��CH3Cl��| A�� | �٢ۢ� | B�� | �ڢۢ� | C�� | ȫ�� | D�� | �ڢ� |
| A�� | ��ȩ | B�� | ��ȩ | C�� | ��ȩ | D�� | 3-����ȩ |
| Ԫ�ر�� Ԫ������ | �� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶��10-10m�� | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 | 0.82 |
| �����ͻ��ϼ� | +2 | +1 | +5 | +7 | +1 | +5 | +3 | |
| -2 | -3 | -1 | -3 |
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 1 | ||||||||
| 2 | ||||||||
| 3 |
 ��
��