��Ŀ����
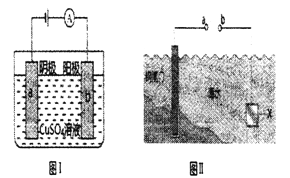
����Ŀ��ȼ�ϵ����Ϊ��ȫ���ܽϺõ�һ�ѧ��Դ�õ��˸���ķ�չ��һ��������(N2H4)Ϊȼ�ϵĻ�����ع���ԭ����ͼ��ʾ������ʱ�����ȶ�����Ⱦ�����ʡ�����˵����ȷ����
A. �����ĵ缫��ӦʽΪ:O2+2H2O+4e-=4OH-
B. ������ÿ����1molN2H4������2molH+ͨ�����ӽ���Ĥ
C. M�����ɵ����ҵ缫����pH����
D. d��������Һ��������ˮ
���𰸡�C
��������A�����������ӵ��ƶ����缫NΪ����������������������ԭ��Ӧ����������c��ͨ�룬�����ĵ缫��ӦʽΪ:O2+4e-- +4H+=2H2O����A����B������Ϊ��������������Ӧ���缫��ӦʽΪ��N2H4-4e-=N2��+4H+����ÿ����lmolN2H4������4molH+ͨ�����ӽ���Ĥ����B����C������B�ķ�����M���ĵ缫��ӦʽΪN2H4-4e-=N2��+4H+��M�����ɵ����ҵ缫����pH���ͣ���C��ȷ��D����Ӧ���ܷ�ӦΪ������������Ӧ���ɵ�����ˮ�����������Һ�����ԣ�d��������Һ����������Һ����D����ѡC��

����Ŀ����֪��I2��2 S2O32-![]() S4O62-��2I-��������ʵ��ܶȻ��������±���
S4O62-��2I-��������ʵ��ܶȻ��������±���
���� | Cu(OH)2 | Fe(OH)3 | CuCl | CuI |
Ksp | 2.2��10-20 | 2.6��10-39 | 1.7��10-7 | 1.3��10-12 |
��1��ij����CuCl2��Һ�к���������FeCl3��Ϊ�õ�������CuCl22H2O���壬����________������pH��4��ʹ��Һ�е�Fe3��ת��ΪFe(OH)3��������ʱ��Һ�е�c(Fe3��)��_____________�����˺�������Һ����������Ũ���ᾧ���ɵõ�CuCl22H2O���塣
��2���ڿ�����ֱ�Ӽ���CuCl22H2O����ò���������ˮCuCl2��ԭ����________________�����û�ѧ����ʽ��ʾ������CuCl22H2O����õ�������ˮCuCl2�ĺ���������_______________��
��3��ijѧϰС��������ӵ��������ⶨ����CuCl22H2O�������������������I-������Ӧ�������������ʣ��Ĵ��ȣ��������£�ȡ0.36 g��������ˮ���������KI���壬��ַ�Ӧ�����ɰ�ɫ��������0.1000 mol/L Na2S2O3����Һ�ζ�������ζ��յ�ʱ������Na2S2O3����Һ20.00 mL����֪��CuCl2��Һ��KI��Ӧ�����ӷ���ʽΪCu2+ + 4I- = 2CuI�� + I2
�ٿ�ѡ��___________���ζ�ָʾ�����ζ��յ��������_________________��
�ڸ�������CuCl22H2O�������ٷ���Ϊ___________________________��