��Ŀ����
����Ŀ��ʵ����Ҫ����480 mL 0.2 mol/L HCl��Һ����ش��������⣺
(1)������Һʱ�IJ����У��ټ��� ���ܽ� ��ϴ�� ��(�ָ����º�)��Һ �ݶ��� ��ҡ�� �߳�������ȷ��˳����___________��
A. �٢ڢۢܢݢޢ� B. �٢ߢڢܢۢݢ� C. �٢ߢڢܢۢޢ�
(2)���ƹ����в���Ҫʹ�õĻ�ѧ������____________(��ѡ�����ĸ)��
A. �ձ� B. 500 mL����ƿ C. ©�� D. ��ͷ�ι� E. ������
(3)������HCl��Һ��ȡ��10 mL��Һ����ԭ��Һ��ȣ����ᷢ���仯����_________��
A. ��Һ��HCl�����ʵ��� B. ��Һ��HCl�����ʵ���Ũ��
C. ��Һ��Cl-����Ŀ D. HCl������
(4)������������Һ�Ĺ����У����������HCl��Һ���ʵ���Ũ���к�Ӱ��(����ƫ��������ƫ������������Ӱ����)
��ת����Һ��δϴ���ձ��Ͳ�������ֱ�Ӷ���___________��
������ƿ������ϴ�Ӻ������������ˮ��___________��
�۶���ʱ��ˮδ�ӵ��̶�___________��
(5)�������ʱ��ˮ��������ƿ�Ŀ̶��ߣ���Ҫ��ȡ��ʩ��__________��
���𰸡�B C ACD ƫ�� ��Ӱ�� ƫ�� ������������
��������
(1)��������һ�������һ�����ʵ���Ũ����Һ�IJ�������
(2)����������Һ���ѡ����Ҫ����ƿ�����������һ�����ʵ���Ũ����Һ��һ�㲽��ѡ����Ҫ��������
(3)������Һ���о�һ�ԣ�����Һ������أ����n=c��V��m=n��M�����жϣ�
(4)������Һ�����ʵ���Ũ�ȶ���ʽc=![]() ����ʵ����
����ʵ����
(5)��������ƿ��ȷ����һ�����ʵ���Ũ�ȵ���Һ������ȷ��ʵ�����������
(1)��ʵ����������һ�����һ�����ʵ���Ũ����Һ�IJ����Ǽ��㡢����(����ȡ)���ܽ�(��ϡ��)����ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ���˲���˳������ű�ʾΪ�٢ߢڢܢۢݢޣ�����ѡ����B��
(2)����һ�����ʵ���Ũ����ҺҪʹ��һ����������ƿ��ѡ�������ı����������������Ҫʹ��500 mL������ƿ��������Һ��һ�㲽�裺���㡢����(����ȡ)���ܽ�(��ϡ��)����ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ��������ᣬҪʹ����Ͳ��ȡŨ���ᣬȻ�����ձ��н���ϡ�ͣ��ò���������ٽ����ʵ��ܽ⣬��������ƿ����ҺʱҪʹ�ò������������������ʱҪʹ�ý�ͷ�ιܣ��ʲ�ʹ�õ�������©�����ʺ���ѡ����C��
(3)��Һ��Ũ������ȡ��Һ������أ����Դ�����HCl��Һ��ȡ��10 mL��Һ����Һ��Ũ�Ȳ��䣬��������Һ�������С���������ʵ���Ũ�ȶ���ʽ��֪n=c��V����Һ�����С��������HCl�����ʵ������٣�����Һ�е��������Cl-����ĿҲ��С������m=n��M��֪�����ʵ����ʵ������٣������Һ�к��е����ʵ�����Ҳ��С���ʷ����仯��ѡ����ACD��
(4)��ת����Һ��δϴ���ձ��Ͳ�������ֱ�Ӷ��ݣ��ᵼ�����ʵ����ʵļ��٣�����Ũ�ȶ���ʽ��֪�����ʵ����ʵ�����С������Һ������䣬����ʹ���Ƶ���ҺŨ��ƫ�ͣ�
������ƿ������ϴ�Ӻ������������ˮ�����ڲ�Ӱ�����ʵ����ʵ����������Һ���������˶�������Һ��Ũ����Ӱ�죻
�۶���ʱ��ˮδ�ӵ��̶ȣ�����Һ�����ƫС���������ʵ����ʵ������䣬������յ���������Һ��Ũ��ƫ�ߣ�
(5)�������ʱ��ˮ��������ƿ�Ŀ̶��ߣ�ʹ��Һ�����ƫ������Һ��Ũ��ƫ�ͣ���Ҫ��ȡ��ʩ�ǽ�����Һ����������������Һ��

 Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д� ������������Ծ�ϵ�д�
������������Ծ�ϵ�д�����Ŀ��(1)�Ҵ�(C2H5OH)��δ����ȼ������ѡ������Һ��ȼ�ϣ�����������ɫֲ��Ľո���ȡ��1.0g�Ҵ���ȫȼ������Һ̬ˮ�ų�1.367kJ��������ʾ�Ҵ�ȼ���ȵ��Ȼ�ѧ����ʽ_____________��
(2)�Ͽ�1molAB(g)�����еĻ�ѧ��ʹ��ֱ�������̬Aԭ�Ӻ���̬Bԭ�������յ�������ΪA��B���ļ��ܡ��±��г���һЩ��ѧ���ļ���E��
��ѧ�� | H-H | O=O | O-H |
E/kJ��mol-1 | 436 | x | 463 |
��ش��������⣺
����ͼ��ʾij��Ӧ�������仯��ϵ����˷�ӦΪ________(����ȡ����ȡ�)��Ӧ�����Ц�H��__________(�ú���a��b�Ĺ�ϵʽ��ʾ)��
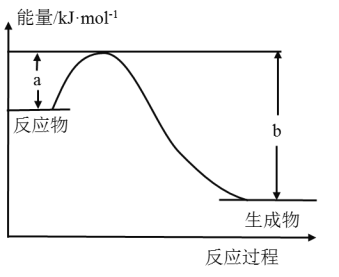
����ͼʾ�б�ʾ��ӦH2(g)��![]() O2(g)=H2O(g)��H����241.8kJ��mol��1����b��_______kJ��mol��1��x��________��
O2(g)=H2O(g)��H����241.8kJ��mol��1����b��_______kJ��mol��1��x��________��