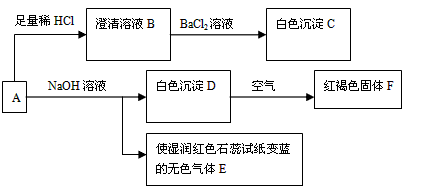
��Ŀ����
��������AΪdz����ɫ�ᾧ����һ����Ҫ�Ļ�ѧ�Լ����������ۺϴ�����A����Һ��������ת����ϵ���ش��������⣺
��1����ɫ����C�Ļ�ѧʽΪ______����ɫ����E�ĵ���ʽΪ______��
��2����Dת����ΪF�Ļ�ѧ��Ӧ����ʽΪ______��
��3����ȡһ����A�ľ��壬���ⶨ��ᾧˮ����ԼΪ27.6%��������ȫ����ˮ�����Һ����������ת�����ɵõ���C��F�����ʵ���֮��Ϊ2��1���ɴ˿�ȷ��A�Ļ�ѧʽΪ______��
��4������ת����ϵ�У�����ϡ�������ϡ���ᣬ______����ܡ����ܡ���ͨ����ת����ϵȷ��A����ɣ�������______��Ҫȷ�Ƴ�C����������Ҫ�ظ����и����ȴ�������IJ�����Ŀ����______��
��5��A�ı���Һ�������ڱ궨���������Һ�ȣ���д���ڸ��������Һ�еμ�A�ı���Һʱ�������������ӷ�Ӧ����ʽ������������ת�������______��

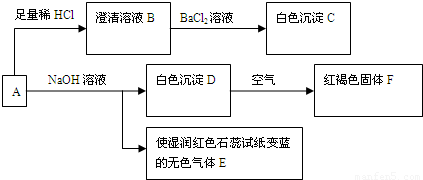
��1����ɫ����C�Ļ�ѧʽΪ______����ɫ����E�ĵ���ʽΪ______��
��2����Dת����ΪF�Ļ�ѧ��Ӧ����ʽΪ______��
��3����ȡһ����A�ľ��壬���ⶨ��ᾧˮ����ԼΪ27.6%��������ȫ����ˮ�����Һ����������ת�����ɵõ���C��F�����ʵ���֮��Ϊ2��1���ɴ˿�ȷ��A�Ļ�ѧʽΪ______��
��4������ת����ϵ�У�����ϡ�������ϡ���ᣬ______����ܡ����ܡ���ͨ����ת����ϵȷ��A����ɣ�������______��Ҫȷ�Ƴ�C����������Ҫ�ظ����и����ȴ�������IJ�����Ŀ����______��
��5��A�ı���Һ�������ڱ궨���������Һ�ȣ���д���ڸ��������Һ�еμ�A�ı���Һʱ�������������ӷ�Ӧ����ʽ������������ת�������______��
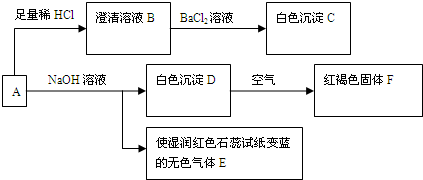
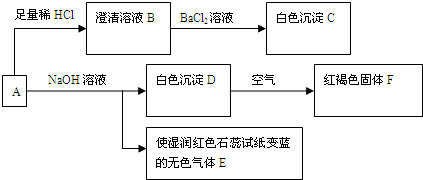
A�����ᷴӦҲ���ԺͼӦ����Ӧ���ɰ�ɫ������ʹʪ��ĺ�ɫʯ����ֽ�����������ǰ�����˵��A�к�笠����ӣ���ɫ�����ڿ����з������ɺ��ɫ������˵������������������DΪ������������˵��A�����������ӣ�A���������ᷴӦ�õ�������ҺB�������Ȼ�����Һ���ɰ�ɫ������˵��A�к�����������ӣ���������AΪdz����ɫ�ᾧ���е�����Ϊ笠����ӡ��������ӡ���������ӣ��ƶϳ����ʷֱ�Ϊ��A������笠����ӡ��������ӡ���������ӣ�B������������ӵ���Һ��C��BaSO4��D��4Fe��OH��2 E��NH3 F��Fe��OH��3
��1�������ƶϰ�ɫ����CΪ��BaSO4����ɫ����E�ǰ����������ĵ���ʽΪ��
���ʴ�Ϊ��BaSO4
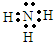
��
��2��D��Fe��OH��2ת����ΪFFe��OH��3�Ļ�ѧ��Ӧ����ʽΪ��4Fe��OH��2+O2+2H2O=4Fe��OH��3���ʴ�Ϊ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��3����ȡһ����A�ľ��壬���ⶨ��ᾧˮ����ԼΪ27.6%��������ȫ����ˮ�����Һ����������ת�����ɵõ���BaSO4��Fe��OH��3�����ʵ���֮��Ϊ2��1��˵��A�к�����������Ӻ���������2��1�����ݻ����ﻯ�ϼ۴�����Ϊ0����笠����ӣ��������ӣ����������=2��1��2����NH4��2Fe��SO4��2 ������������A��ѧʽΪ����NH4��2Fe��SO4��2 ?xH2O��A�ľ��壬���ⶨ��ᾧˮ����ԼΪ27.6%��
��100%=27.6%��x=6������A�Ļ�ѧʽΪ����NH4��2Fe��SO4��2 ?6H2O���ʴ�Ϊ����NH4��2Fe��SO4��2 ?6H2O��
��4������ϡ�������ϡ���ᣬ�������ǿ�����ԣ�����ȷ����Ԫ�صĴ���״̬������ͨ����ת����ϵȷ��A����ɣ���Ϊ����ȷ�����������λ����������Σ�Ҫȷ�Ƴ�C��BaSO4������������Ҫ�ظ����и����ȴ�������IJ�����Ŀ���dz��׳�ȥC��BaSO4�������ˮ�֣�
�ʴ�Ϊ�����ܣ���Ϊ����ȷ�����������λ����������Σ�Ŀ���dz��׳�ȥC��BaSO4�������ˮ�֣�
��5�����������Һ�еμ�A������NH4��2Fe��SO4��2 ?6H2O���ı���Һʱ�����������Һ����A�е� �������ӷ���������ԭ��Ӧ����Ӧ�����ӷ���ʽΪ��
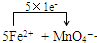
+8H+=5Fe3++Mn2++4H2O��
�ʴ�Ϊ��
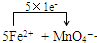
+8H+=5Fe3++Mn2++4H2O��
��1�������ƶϰ�ɫ����CΪ��BaSO4����ɫ����E�ǰ����������ĵ���ʽΪ��
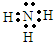
���ʴ�Ϊ��BaSO4
��
��2��D��Fe��OH��2ת����ΪFFe��OH��3�Ļ�ѧ��Ӧ����ʽΪ��4Fe��OH��2+O2+2H2O=4Fe��OH��3���ʴ�Ϊ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��3����ȡһ����A�ľ��壬���ⶨ��ᾧˮ����ԼΪ27.6%��������ȫ����ˮ�����Һ����������ת�����ɵõ���BaSO4��Fe��OH��3�����ʵ���֮��Ϊ2��1��˵��A�к�����������Ӻ���������2��1�����ݻ����ﻯ�ϼ۴�����Ϊ0����笠����ӣ��������ӣ����������=2��1��2����NH4��2Fe��SO4��2 ������������A��ѧʽΪ����NH4��2Fe��SO4��2 ?xH2O��A�ľ��壬���ⶨ��ᾧˮ����ԼΪ27.6%��
| xH2O |
| (NH4)2Fe(SO4)2?xH2O |
��4������ϡ�������ϡ���ᣬ�������ǿ�����ԣ�����ȷ����Ԫ�صĴ���״̬������ͨ����ת����ϵȷ��A����ɣ���Ϊ����ȷ�����������λ����������Σ�Ҫȷ�Ƴ�C��BaSO4������������Ҫ�ظ����и����ȴ�������IJ�����Ŀ���dz��׳�ȥC��BaSO4�������ˮ�֣�
�ʴ�Ϊ�����ܣ���Ϊ����ȷ�����������λ����������Σ�Ŀ���dz��׳�ȥC��BaSO4�������ˮ�֣�
��5�����������Һ�еμ�A������NH4��2Fe��SO4��2 ?6H2O���ı���Һʱ�����������Һ����A�е� �������ӷ���������ԭ��Ӧ����Ӧ�����ӷ���ʽΪ��
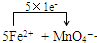
+8H+=5Fe3++Mn2++4H2O��
�ʴ�Ϊ��
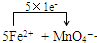
+8H+=5Fe3++Mn2++4H2O��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
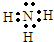
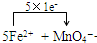 +8H+=5Fe3++Mn2++4H2O
+8H+=5Fe3++Mn2++4H2O