��Ŀ����
��������AΪdz����ɫ�ᾧ����һ����Ҫ�Ļ�ѧ�Լ����������ۺϴ�����A����Һ��������ת����ϵ
�ش��������⣺
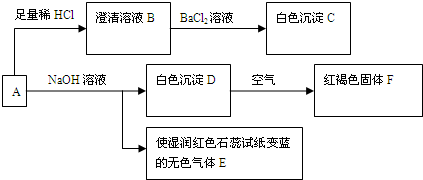
��1����ɫ����C�Ļ�ѧʽΪ_____________����ɫ����E�ĵ���ʽΪ_________________��
��2����Dת����ΪF�Ļ�ѧ��Ӧ����ʽΪ_________________��
��3����ȡһ����A�ľ��壬���ⶨ��ᾧˮ����ԼΪ27.6%��������ȫ����ˮ�����Һ����������ת�����ɵõ���C��F�����ʵ���֮��Ϊ2�U1���ɴ˿�ȷ��A�Ļ�ѧʽΪ_______________��
��4������ת����ϵ�У�����ϡ�������ϡ���ᣬ_________����ܡ����ܡ���ͨ����ת����ϵȷ��A����ɣ�������_______________________��Ҫȷ�Ƴ�C����������Ҫ�ظ����и����ȴ�������IJ�����Ŀ����_____________________��
��5��A�ı���Һ�������ڱ궨���������Һ�ȡ���д���ڸ��������Һ�еμ�A�ı���Һʱ�������������ӷ�Ӧ����ʽ������������ת�������________________________��
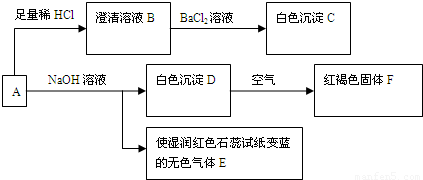
��1����ɫ����C�Ļ�ѧʽΪ_____________����ɫ����E�ĵ���ʽΪ_________________��
��2����Dת����ΪF�Ļ�ѧ��Ӧ����ʽΪ_________________��
��3����ȡһ����A�ľ��壬���ⶨ��ᾧˮ����ԼΪ27.6%��������ȫ����ˮ�����Һ����������ת�����ɵõ���C��F�����ʵ���֮��Ϊ2�U1���ɴ˿�ȷ��A�Ļ�ѧʽΪ_______________��
��4������ת����ϵ�У�����ϡ�������ϡ���ᣬ_________����ܡ����ܡ���ͨ����ת����ϵȷ��A����ɣ�������_______________________��Ҫȷ�Ƴ�C����������Ҫ�ظ����и����ȴ�������IJ�����Ŀ����_____________________��
��5��A�ı���Һ�������ڱ궨���������Һ�ȡ���д���ڸ��������Һ�еμ�A�ı���Һʱ�������������ӷ�Ӧ����ʽ������������ת�������________________________��
��1��BaSO4��
��2��4Fe(OH)2+O2+2H2O = 4Fe(OH)3
��3��(NH4)2Fe(SO4)2��6H2O
��4�����ܣ���Ϊ����ȷ�����������λ����������Σ����׳�ȥC�����ˮ��
��5��

��2��4Fe(OH)2+O2+2H2O = 4Fe(OH)3
��3��(NH4)2Fe(SO4)2��6H2O
��4�����ܣ���Ϊ����ȷ�����������λ����������Σ����׳�ȥC�����ˮ��
��5��


��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
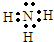
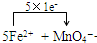 +8H+=5Fe3++Mn2++4H2O
+8H+=5Fe3++Mn2++4H2O