��Ŀ����
8�� ��ѧС���������������������װ�ã���ͼ�����û������Ʊ�����ϩ��
��ѧС���������������������װ�ã���ͼ�����û������Ʊ�����ϩ����֪��

| �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� | |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |
�ٵ���B���˵�������е�������������
���Թ�C���ڱ�ˮԡ�е�Ŀ����ʹ����ϩҺ�������ٻӷ���
��2���ٻ���ϩ��Ʒ�к��л������������������ʵȣ����뱥��ʳ��ˮ������ֹ���ֲ㣬����ϩ���ϲ㣨��ϡ����¡�������Һ����C����ѡ����ĸ��ϴ�ӣ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
�������ռ���Ʒʱ�����Ƶ��¶�Ӧ��83�����ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����C����ѡ����ĸ����
A������ʱ��70�濪ʼ�ռ���Ʒ
B��������ʵ����������
C���Ʊ���Ʒʱ���������Ʒһ��������
���� ��1���ٸ�������ϩʵ���֪ʶ������װ��A�����Ƭ�������Ƿ�ֹ���У��������ɵĻ���ϩ�ķе�Ϊ83�棬Ҫ�õ�Һ̬����ϩ������B���˵���������������ã����ڻ���ϩ������
�ڱ�ˮԡ��Ŀ���ǽ��ͻ���ϩ�������¶ȣ�ʹ��Һ����
��2���ٻ���ϩ�������Ȼ�����Һ�����ܶȱ�ˮС���ֲ��ϩ���ϲ㣬���ڷ�Һ��ϩ��Ʒ�л�������������ͻ��������ᴿ����ʱ��c��Na2CO3��Һ��ϴ�ӿɳ�ȥ�
�ڸ��ݱ������ݿ�֪����ֻ���ϩ�ķе�Ϊ83�棻
A������ǰ�ռ�����Ʒ�л������ʣ�ʵ�ʲ����������۲�����
B����ȡ�Ļ���ϩ���ʵ�������ʵ���ƵõĻ���ϩ��Ʒ�����������۲�����
C���ֲ�Ʒ�л��л����������²ⶨ���ĵĻ������������ƵõĻ���ϩ��Ʒ�����������۲�����
��� �⣺��1���ٸ�������ϩʵ���֪ʶ������װ��A�����Ƭ�������Ƿ�ֹ���У��������ɵĻ���ϩ�ķе�Ϊ83�棬Ҫ�õ�Һ̬����ϩ������B���˵���������������ã����ڻ���ϩ������
�ʴ�Ϊ��������
�ڱ�ˮԡ��Ŀ���ǽ��ͻ���ϩ�������¶ȣ�ʹ��Һ����
�ʴ�Ϊ��ʹ����ϩҺ�������ٻӷ���
��4���ٻ���ϩ�����࣬�������Ȼ�����Һ�����ܶȱ�ˮС�������á��ֲ��ϩ���ϲ㣬���ڷ�Һ��ϩ��Ʒ�л�������������ͻ����������룺�Ʊ����������ᴿ����ʱ��C��Na2CO3��Һ��ϴ�ӿɳ�ȥ�
�ʴ�Ϊ���ϣ�C��
�ڸ��ݱ������ݿ�֪����ֻ���ϩ�ķе�Ϊ83�棬���ռ���ƷӦ�����¶���83�����ң�
A������ʱ��70�濪ʼ�ռ���Ʒ����ǰ�ռ�����Ʒ�л������ʣ�ʵ�ʲ����������۲�������A����
B��������ʵ���������ˣ���ȡ�Ļ���ϩ�����ʵ�������ʵ���ƵõĻ���ϩ��Ʒ�����������۲�������B����
C�����ֲ�Ʒ�л��л����������²ⶨ���ĵĻ������������ƵõĻ���ϩ��Ʒ�����������۲�������C��ȷ������ѡC��
�ʴ�Ϊ��83�棻C��
���� ���⿼���������������Ʊ����Ի������Ʊ�����ϩ��ʵ�鷽�����ۺϿ��������ʵķ��뷽���������������ķ����ȣ��Ѷ����У�����ѧ�����ʵ�������������

| A�� | 14C��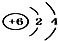 | B�� | 16O��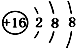 | C�� | Li+��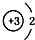 | D�� | H-��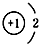 |
��CH3CH2CH2OH��CH3��CH2��2CH3
��CH3��CH2��2CH3��CH3CH��CH3��2
�۱��ӣ���
��CH3CH2Br��CH3CH2OH
��
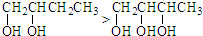
| A�� | �٢� | B�� | �ڢܢ� | C�� | �٢ڢ� | D�� | �ۢܢ� |
| A�� | �����CuO��NO��SO2��H2O | |
| B�� | �NaOH��KOH��Ba��OH��2��Na2CO3 | |
| C�� | ���������Na2O��CaO��Al2O3��Na2O2 | |
| D�� | ����ʣ�KNO3��Cl2��HCl��BaSO4 |
| A�� | ���ݶ��������ɽ���ɢϵ����Ϊ��Һ����������Һ | |
| B�� | PM2.5����ֱ��ԼΪ2.5��10-6 m����ɢ�ڿ������γ����ܽ� | |
| C�� | ���ά������ϩ����֬���Ǹ߷��ӻ����� | |
| D�� | �������γ���������β���ŷ��кܴ��ϵ |
| A�� | �ô���Һ��ϴ�ζ��õ���ƿ | |
| B�� | �����£�ijͬѧ��pH��ֽ���KCl��Һ��pHԼΪ7.0 | |
| C�� | ����Fe2��SO4��3��Һʱ���Ȱ�Fe2��SO4��3��������Ũ�����ϡ�� | |
| D�� | �ü�ʽ�ζ���ȷ��ȡKMnO4��Һ�����Ϊ21.50mL |
| A�� | һ���¶Ⱥ�ѹǿ�£���̬���ʵ������Ҫ�ɹ�������ķ��ӵĴ�С���� | |
| B�� | ��1 L 2 mol/L��H2SO4��Һ��ȡ��0.5 L������Һ�������ӵ�Ũ��Ϊ4mol/L | |
| C�� | ͬ��ͬѹ�£�30mLA2�����10mL B2����ǡ����ȫ��Ӧ����20mLC���壬��C��ѧʽΪ A3 B��B A3 | |
| D�� | ͬ��ͬѹ���κ�����ķ��Ӽ���뼸����� |
| A�� | H2O������ʱ��Cu��H2O�γɦҼ� | B�� | H2O����λ��������NH3 | ||
| C�� | �Ҵ��ɼ�С���Ӿ�����ܽ�� | D�� | Cu��NH3��4SO4��BaCl2�а�ɫ�������� |
 ��֪A��B��C��D��E����Ԫ�����ڱ��е�ǰ36��Ԫ�أ����ǵ�ԭ��������������Bԭ�ӻ�̬ʱPԭ�ӹ������3��δ�ɶԵ��ӣ����������������۴�����Ϊ2��C�ļ۲�����Ų�ʽΪns2npn+2�����⻯����ͬ��Ԫ�����γɵ��⻯���зе��������AC2Ϊ�Ǽ��Է��ӣ�DԪ�ص�ԭ�Ӻ����20�ֲ�ͬ�˶�״̬�ĵ��ӣ�E��Ԫ�����ڱ��������ڵ�9��Ԫ�أ���ش�
��֪A��B��C��D��E����Ԫ�����ڱ��е�ǰ36��Ԫ�أ����ǵ�ԭ��������������Bԭ�ӻ�̬ʱPԭ�ӹ������3��δ�ɶԵ��ӣ����������������۴�����Ϊ2��C�ļ۲�����Ų�ʽΪns2npn+2�����⻯����ͬ��Ԫ�����γɵ��⻯���зе��������AC2Ϊ�Ǽ��Է��ӣ�DԪ�ص�ԭ�Ӻ����20�ֲ�ͬ�˶�״̬�ĵ��ӣ�E��Ԫ�����ڱ��������ڵ�9��Ԫ�أ���ش�