��Ŀ����
��12�֣����̿����Ҫ�ɷ���̼���̣����� Fe2O3��FeO ��CaO��MgO �ȳɷ֡�ij�������÷����ᣨ��������Լ20%�������̿��Ʊ�MnCl2��4H2O��106��ʱʧȥһ���ӽᾧˮ��198��ʱʧȥȫ���ᾧˮ������ˮ������ֹ����������£�

��1������������þ����̼���̷�Ӧ�Ļ�ѧ����ʽΪ��_______________________________��
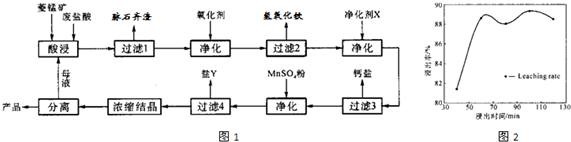
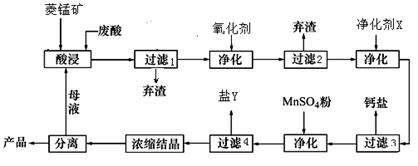
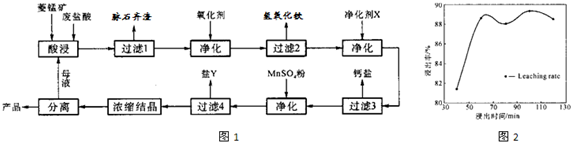
��2�����ʱ������ʱ����̽����ʵ�Ӱ������ͼ����ҵ���õ��ǽ�ȡ60min�������ԭ���ǣ�____________________________________________________��
��3��ͼ�С�������X��������________________________��
��4������4��õ���Y������Ҫ�ɷ���__________________________��
��5��Ũ���ᾧʱ������һ���־�Ĥ����ֹͣ���ȣ���ԭ���ǣ�_______________________��
��1��MnCO3+2HCl=MnCl2+CO2��+H2O��3�֣�
��2��60min�����ӳ�����ʱ�����������ɱ��������������������ԡ���3�֣�[��Դ:ѧ&��&��]
��3��MnCO3��2�֣�
��4������þ��MgSO4��7H2O����2�֣�
��5��ˮ�ݺ���ʱ�����յõ��Ŀ�����ʧȥ���ֽᾧˮ���Ȼ��̡���2�֣�
����������1��̼���̺����ᷴӦ���������Ȼ��̡�ˮ��CO2����ѧ����ʽΪ
MnCO3+2HCl=MnCl2+CO2��+H2O��
��2������ͼ���֪��60min�����ӳ�����ʱ�����������������ԣ����һ������������ɱ���
��3����Ϊ���������µ����ʣ����ԡ�������X����MnCO3��
��4������ת��ͼ��֪����ʱ������Һ�е������ӳ����������⣬��Ҫ����þ���ӣ�����Y������þ��
��5��MnCl2��4H2O�ڼ���ʱ����ʧȥ�ᾧˮ�����²�Ʒ������

 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�