��Ŀ����
�������Ļ�������������������������Ҫ����;��
(1)�ۺ�������(���PFS)�Ļ�ѧʽΪ[Fe(OH)n(SO4)(3-n)/2]m������ˮ��������������PFS��ˮ�����γ���״��������������ӡ���PFS����Ԫ�ؼ�̬��ͬ���������ӵĵ����Ų�ʽΪ____________��
(2)������������K4[Fe(CN)6]��������Ӱ�����û������д��ڵĻ�ѧ��������___________��
A�����Ӽ� B�����ۼ� C�������� D����λ�� E�����
CN-��̼ԭ�ӵ��ӻ����������_________��д��һ����CN-��Ϊ�ȵ���������ӵĻ�ѧʽ________��
(3)���Ȼ����ڳ�����Ϊ���壬�۵�304�棬�е�316�棬��300�����Ͽ�������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж����Ȼ�������Ϊ___________���塣
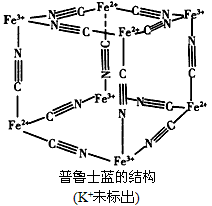
(4)��³ʿ��������Ⱦ�ϣ����Ľṹ��ͼ��ʾ����³ʿ���У�n(K+)��n(Fe3+)��n(Fe2+)��n(CN-)=_____��
(1)�ۺ�������(���PFS)�Ļ�ѧʽΪ[Fe(OH)n(SO4)(3-n)/2]m������ˮ��������������PFS��ˮ�����γ���״��������������ӡ���PFS����Ԫ�ؼ�̬��ͬ���������ӵĵ����Ų�ʽΪ____________��
(2)������������K4[Fe(CN)6]��������Ӱ�����û������д��ڵĻ�ѧ��������___________��
A�����Ӽ� B�����ۼ� C�������� D����λ�� E�����
CN-��̼ԭ�ӵ��ӻ����������_________��д��һ����CN-��Ϊ�ȵ���������ӵĻ�ѧʽ________��
(3)���Ȼ����ڳ�����Ϊ���壬�۵�304�棬�е�316�棬��300�����Ͽ�������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж����Ȼ�������Ϊ___________���塣
(4)��³ʿ��������Ⱦ�ϣ����Ľṹ��ͼ��ʾ����³ʿ���У�n(K+)��n(Fe3+)��n(Fe2+)��n(CN-)=_____��
(1)1s22s22p63s23p63d5��[Ar]3d5
(2)ABD��sp��C22-
(3)����
(4)1:1:1:6
(2)ABD��sp��C22-
(3)����
(4)1:1:1:6

��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
 ��2011?�Ͼ�һģ���������Ļ�������������������������Ҫ����;��
��2011?�Ͼ�һģ���������Ļ�������������������������Ҫ����;��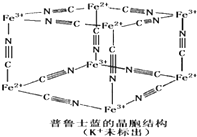 ��1���������Ļ�������������������������Ҫ����;��
��1���������Ļ�������������������������Ҫ����;��
 Ҫ����;��
Ҫ����;�� ���������ؽ������ӡ���PFS������ԭ��δ�ɶԵ�����Ϊ__ ����
���������ؽ������ӡ���PFS������ԭ��δ�ɶԵ�����Ϊ__ ���� ____ ��
____ ��