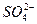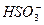��Ŀ����
����Ȼ���еĿ����У�CuSO4�ɽ�FeS2����������Cu2S��SO42����Fe2+�����б�ʾ�÷�Ӧ�����ӷ���ʽ�У���ȷ���� �� ��
| A��14Cu2++5S22��+12H2O=7Cu2S+3SO42��+24H+ |
| B��14Cu2++5FeS2+28OH��=7Cu2S+3SO42��+5Fe2++14H2O |
| C��14Cu2+ +5FeS2+24OH��=7Cu2S+3SO42��+5Fe2++12H2O |
| D��14Cu2++5FeS2+12H2O=7Cu2S+3SO42��+5Fe2++24H+ |
D
��������ƽ�ã�14Cu2+ +5FeS2��7Cu2S+3SO42��+5Fe2+����ʽ��߹���28����λ����ɣ��ұ߹���4����λ����ɡ��������ǵ���غ㣬������ʽ�����24��OH����Ҳ�����ұ���24��H+����Cu2+��Fe2+�ڼ��Ի����л��γɳ���������ӦΪ���ԣ���Ӧ����ʽ�ұ���24��H+���ã�14Cu2++5FeS2��7Cu2S+3SO42��+5Fe2++24H+������ù۲취������ƽ��14Cu2++5FeS2+12H2O=7Cu2S+3SO42��+5Fe2++24H+������ѡ���У�A�ĵ�ɲ��غ㣬B��C�Ľ��ʶ��Ǽ��ԣ���˶����ԡ��𰸣�D��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ���������⡣
���������⡣ �����ܽ���ˮ�Ƶã�����εĻ�ѧʽΪ ��
�����ܽ���ˮ�Ƶã�����εĻ�ѧʽΪ ��  ͬʱ��һ����⻬������С����������Һ���룬����ձ��л���ע����H2SO4��ҺͬŨ�ȵ�Ba(OH)2��Һ��ǡ����ȫ��Ӧ���Իش��������⣺
ͬʱ��һ����⻬������С����������Һ���룬����ձ��л���ע����H2SO4��ҺͬŨ�ȵ�Ba(OH)2��Һ��ǡ����ȫ��Ӧ���Իش��������⣺
 ��Mg2+��Fe3+��Al3+��
��Mg2+��Fe3+��Al3+��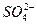 �����ӣ��������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ��ʾ���ɴ˿�֪������Һ�п϶����е���������_____________���Ҹ������ӵ����ʵ���֮��Ϊ_____________���϶������е���������_____________��������ܺ��е�ij�������ӵ�ʵ�鷽����____________________________________________________��
�����ӣ��������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ��ʾ���ɴ˿�֪������Һ�п϶����е���������_____________���Ҹ������ӵ����ʵ���֮��Ϊ_____________���϶������е���������_____________��������ܺ��е�ij�������ӵ�ʵ�鷽����____________________________________________________��
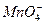
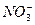 ��Cl-��
��Cl-��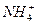
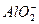 ��
��