��Ŀ����
����Ŀ�������ᣨ��ʽ��ϩ���ᣩ��Fe2+�γɵ����������������ֳơ���Ѫ����������������ȱ����ƶѪ�������Ǻϳɸ���������һ�ֹ���·�ߣ�

�ش��������⣺
��1��A�Ļ�ѧ����Ϊ ����A����B�ķ�Ӧ����Ϊ ��
��2��C�Ľṹ��ʽΪ ��
��3��������Ľṹ��ʽΪ ��
��4�����鸻Ѫ�����Ƿ���Fe3+��ʵ����������� ��
��5��������Ϊ��Ԫ���ᣬ1mol����������������NaHCO3��Һ��Ӧ�ɷų� L CO2
����������������ͬ���칹���У�ͬΪ��Ԫ����Ļ��� �� ��д���ṹ��ʽ��
���𰸡�
��1�������� ȡ����Ӧ ��ÿ��2�֣���4�֣�
��2��![]() ��2�֣�
��2�֣�
��3��![]() ��2�֣�
��2�֣�
��4��ȡ������Ѫ��������ϡ�����ܽ⣬�ٵμ�KSCN��Һ������Һ��Ѫ��ɫ�����Ʒ�к���Fe3+����
֮�����ޡ� ��2�֣�
��5��44.8 ��1�֣�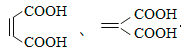
��������
�������������������������ȡ����Ӧ����B��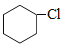 ��B������ȥ��Ӧ���ɻ���ϩ������ϩ����ӳ�����C��C��
��B������ȥ��Ӧ���ɻ���ϩ������ϩ����ӳ�����C��C��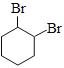 ��C����ȥ����
��C����ȥ����![]() ��
��![]() ����������ȡ����Ӧ����
����������ȡ����Ӧ����![]() ��
��![]() ����������Ӧ����
����������Ӧ����![]() ������ȥ���к͵�
������ȥ���к͵�![]() ���ɽ����ữ��
���ɽ����ữ��![]()
��1��![]() �Ļ�ѧ����Ϊ ������ ����
�Ļ�ѧ����Ϊ ������ ����![]() ����
����![]() �ķ�Ӧ����Ϊ ȡ����Ӧ ��
�ķ�Ӧ����Ϊ ȡ����Ӧ ��
��2��C�Ľṹ��ʽΪ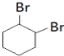 ��
��
��3��������Ľṹ��ʽΪ![]() ��
��
��4�����鸻Ѫ�����Ƿ���Fe3+��ʵ����������� ȡ������Ѫ��������ϡ�����ܽ⣬�ٵμ�KSCN��Һ������Һ��Ѫ��ɫ�����Ʒ�к���Fe3+����֮������ ��
��5��������Ϊ��Ԫ���ᣬ1mol����������������NaHCO3��Һ��Ӧ�ɷų� 44.8 LCO2����������������ͬ���칹���У�ͬΪ��Ԫ����Ļ��� 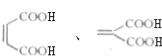 ��
��

 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�����Ŀ��ij��ѧС��̽������������N03 -��S042-��Fe3+��������������ǿ�����������ʵ�飨�г���������ȥ��װ�õ��������Ѽ���)�� (���������Է�Ӧ��Ӱ�죩

ʵ���¼����:
ʵ�� ��� | ʵ����� | '...!ʵ������ |
I | ��Aװ����ͨ��һ��ʱ���SO2���塣. | A��gɫ��Һ���ձ�Ϊdz��ɫ�� |
II | ȡ������Aװ���е����ƣ��ȼ��� KSCN��Һ���ټ���BaCl2��Һ�� | ����KSCN��Һ����Һ����ɫ;�ټ���BaCl2��Һ������ɫ������ |
III | ����a��������ϡHNO3����װ��A �У��رջ���a�� | A��dz��ɫ��Һ���ձ�Ϊ��ɫ�� |
IV | ȡ������Aװ���е���Һ������KSCN ��Һ�� | ��Һ��Ϊ��ɫ�� |
��ش��������⣺
��1��д��Aװ����ͨ�˶����������巢����Ӧ�����ӷ���ʽ ��
��2��ʵ��II�з�����Ӧ�����ӷ���ʽ�� ��
��3��ʵ��III�У�dz��ɫ��Һ��Ϊ��ɫ��ԭ���� ��(��������������
��4��ʵ��IV����A��ͨ���˿�����Һ���Ϸ��������� ��
��5���ۺ�����ʵ��Ì�Ľ�����:�����������£�������ǿ��˳��Ϊ ��


