��Ŀ����
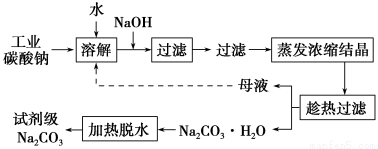
��ҵ̼����(����ԼΪ98%)�к���Ca2����Mg2����Fe3����Cl����SO42-�����ʣ��ᴿ������·���£�
��.̼���Ƶı�����Һ�ڲ�ͬ�¶���������������ͼ��ʾ��

��.�й����ʵ��ܶȻ����£�
���� | CaCO3 | MgCO3 | Ca(OH)2 | Mg(OH)2 | Fe(OH)3 |
Ksp | 4.96��10��9 | 6.82��10��6 | 4.68��10��6 | 5.61��10��12 | 2.64��10��39 |
�ش��������⣺
(1)����NaOH��Һʱ������Ӧ�����ӷ���ʽΪ��_____________________________________��
����Mg2����Fe3������Һ�еμ�NaOH��Һ�������ֳ�����������Һ��pH��8ʱ��c(Mg2��)��c(Fe3��)��________��
(2)�����ȹ�����ʱ���¶�Ӧ������________��
(3)���˴�����ɫ��ѧ���Ƕ����뽫��ĸҺ����������������ʾ����ѭ��ʹ�á��������ʵ�ʹ�ҵ�������Ƿ����________����˵������________________________________________��
(4)��֪��Na2CO3��10H2O(s)=Na2CO3(s)��10H2O(g)����H����532.36 kJ��mol��1
Na2CO3��10H2O(s)=Na2CO3��H2O(s)��9H2O(g)��H����473.63 kJ��mol��1
д��NaCO3��H2O��ˮ��Ӧ���Ȼ�ѧ����ʽ________________________________________��
(1)Fe3����3OH��=Fe(OH)3����MgCO3��2OH��=Mg(OH)2��CO32-��Mg2����2OH��=Mg(OH)��(���������������)��2.125��1021
(2)����36 ��
(3)�����С�����ĸҺ��ѭ��ʹ�ã�����Һ��c(Cl��)��c(SO42-)����������ò���Na2CO3�л�������
(4)Na2CO3��H2O(s)=Na2CO3(s)��H2O(g)��H����58.73 kJ��mol��1
��������(1)���ݱ������ݣ���������֪����NaOH��ҺʱMg2����Fe3���ֱ���OH��������ɳ���Mg(OH)2��Fe(OH)3��(2)�¶�Խ��̼���ƾ�����ܽ��Խ��Ϊ�˼���̼���Ƶ���ʧ����Ӧ���ȹ��ˣ����������������ݣ����¶ȸ���36 �������������١�(3)��ĸҺ����������������ʾ����ѭ��ʹ��ʱ�������Cl����SO42-�����ӵĸ�����ʹ���ò�Ʒ�к������ʡ�
(4)��������֪���Ȼ�ѧ����ʽ��˳����Ϊ�٢������ݸ�˹������������Na2CO3��H2O(s)=Na2CO3(s)��H2O(g)����H����58.73 kJ��mol��1��

���������������ֵ�̼�������(����)��
A������ʳ��ӹ�����
B��ע���Լ�õ�
C���������صġ�������ʳ��
D������ʹ��н��Ϊȼ��
��ѧ��������������������һЩ����ʱ������Һ�������˶��ֱ���Ԫ�أ����䷽���£�����ֲ����������ٵ�������(����)��
���� | K�� | Mg2�� | Ca2�� | NO3- | H2PO4- | SO42- | Zn2�� |
| 1 | 0.25 | 1 | 2 | 1 | 0.25 | 1 |
A.Zn2�� B��SO42- C��Ca2�� D��H2PO4-