��Ŀ����
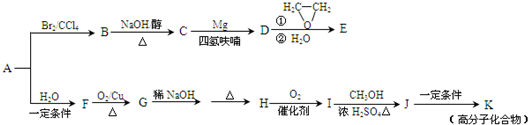
��A��һ����Ҫ�Ļ�������ԭ�ϣ��������������Է�������Ϊ28����ͼ1����AΪԭ�Ϻϳ�ҩ���м���E����֬K��·�ߣ�
��֪��ͼ2��
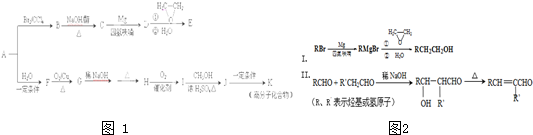
��1��A�й����ŵĽṹ��ʽ��______���л���B������______��
��2��B��C�Ļ�ѧ����ʽΪ______��B���������Ƶ�ˮ��Һ���ȷ�Ӧ���õ����л�������Ҷ��ᷴӦ���ɸ߷��ӻ����д�����ɸ߷��ӻ����ﷴӦ�Ļ�ѧ����ʽ______��
��3��E�ķ���ʽΪC4H8O�����й���E��˵����ȷ����______������ĸ��ţ���
a����������Ʒ�Ӧ b��������4��̼ԭ��һ����ƽ��
c��һ�������£�����Ũ�����ᷴӦ d����CH2=CHCH2OCH2CH3��Ϊͬϵ��
��4��G��H�漰���ķ�Ӧ������______��
��5��I�ķ���ʽΪC4H6O2����ṹ��ʽΪ______��
��6��J��K�Ļ�ѧ����ʽΪ______��
��7��д����E������ͬ�����ŵ�����ͬ���칹��Ľṹ��ʽ��______��������˳���칹��������-OH����˫��̼�ϵĽṹ����
��֪��ͼ2��

��1��A�й����ŵĽṹ��ʽ��______���л���B������______��
��2��B��C�Ļ�ѧ����ʽΪ______��B���������Ƶ�ˮ��Һ���ȷ�Ӧ���õ����л�������Ҷ��ᷴӦ���ɸ߷��ӻ����д�����ɸ߷��ӻ����ﷴӦ�Ļ�ѧ����ʽ______��
��3��E�ķ���ʽΪC4H8O�����й���E��˵����ȷ����______������ĸ��ţ���
a����������Ʒ�Ӧ b��������4��̼ԭ��һ����ƽ��
c��һ�������£�����Ũ�����ᷴӦ d����CH2=CHCH2OCH2CH3��Ϊͬϵ��
��4��G��H�漰���ķ�Ӧ������______��
��5��I�ķ���ʽΪC4H6O2����ṹ��ʽΪ______��
��6��J��K�Ļ�ѧ����ʽΪ______��
��7��д����E������ͬ�����ŵ�����ͬ���칹��Ľṹ��ʽ��______��������˳���칹��������-OH����˫��̼�ϵĽṹ����
��A����Է�������Ϊ28����AӦΪCH2=CH2�����巢���ӳɷ�Ӧ����B��BΪBrCH2CH2Br�����������ƴ���Һ�����������·�����ȥ��Ӧ����C�������Ϣ���֪CΪCH2=CHBr��DΪCH2=CHMgBr��EΪCH2=CHCH2CH2OH��A��ˮ�����ӳɷ�Ӧ����FΪCH3CH2OH��F����������G����GΪCH3CHO������Ϣ���֪HΪCH3CH=CHCHO��H����������I��IΪCH3CH=CHCOOH����״�����������Ӧ����J����JΪCH3CH=CHCOOCH3��J�����Ӿ۷�Ӧ���ɸ߷��ӻ�����K����KΪ
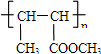
��
��1�������Ϸ�����֪AΪCH2=CH2���л���BΪBrCH2CH2Br�����й�����Ϊ��ԭ�ӣ��ʴ�Ϊ��CH2=CH2����ԭ�ӣ�
��2��BΪBrCH2CH2Br��CΪCH2=CHBr��B����C�ķ���ʽΪ��BrCH2CH2Br+NaOH
CH2=CHBr+NaBr+H2O��
B���������Ƶ�ˮ��Һ���ȷ�Ӧ���õ�HOCH2CH2OH�����Ҷ��ᷴӦ���ɸ߷��ӻ�����ķ���ʽΪ��
HOCH2CH2OH+HOOC-COOH
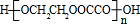
+2��n-1��H2O��
�ʴ�Ϊ��BrCH2CH2Br+NaOH
CH2=CHBr+NaBr+H2O��HOCH2CH2OH+HOOC-COOH
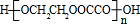
+2��n-1��H2O��
��3��EΪCH2=CHCH2CH2OH������C=C���ܷ����ӳɷ�Ӧ������-OH������Na������Ӧ������������CH2=CHCH2OCH2CH3�ṹ��ͬ������ͬϵ�������ֻ��3��Cԭ����ͬһ��ƽ���ϣ�
�ʴ�Ϊ��ac��
��4������Ϣ���֪G��H�漰���ķ�Ӧ�����мӳɷ�Ӧ����ȥ��Ӧ���ʴ�Ϊ���ӳɷ�Ӧ����ȥ��Ӧ��
��5�������Ϸ�����֪IΪCH3CH=CHCOOH���ʴ�Ϊ��CH3CH=CHCOOH��
��6��JΪCH3CH=CHCOOCH3������̼̼˫�����ɷ����Ӿ۷�Ӧ����Ӧ�ķ���ʽΪ
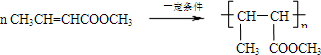
��
�ʴ�Ϊ��
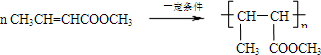
��
��7��EΪCH2=CHCH2CH2OH����E������ͬ�����ŵ�����ͬ���칹��Ϊ
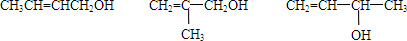
��
�ʴ�Ϊ��
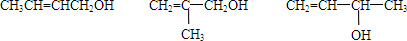
��
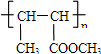
��
��1�������Ϸ�����֪AΪCH2=CH2���л���BΪBrCH2CH2Br�����й�����Ϊ��ԭ�ӣ��ʴ�Ϊ��CH2=CH2����ԭ�ӣ�
��2��BΪBrCH2CH2Br��CΪCH2=CHBr��B����C�ķ���ʽΪ��BrCH2CH2Br+NaOH
| �� |
| �� |
B���������Ƶ�ˮ��Һ���ȷ�Ӧ���õ�HOCH2CH2OH�����Ҷ��ᷴӦ���ɸ߷��ӻ�����ķ���ʽΪ��
HOCH2CH2OH+HOOC-COOH
| һ������ |
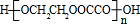
+2��n-1��H2O��
�ʴ�Ϊ��BrCH2CH2Br+NaOH
| �� |
| �� |
| һ������ |
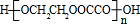
+2��n-1��H2O��
��3��EΪCH2=CHCH2CH2OH������C=C���ܷ����ӳɷ�Ӧ������-OH������Na������Ӧ������������CH2=CHCH2OCH2CH3�ṹ��ͬ������ͬϵ�������ֻ��3��Cԭ����ͬһ��ƽ���ϣ�
�ʴ�Ϊ��ac��
��4������Ϣ���֪G��H�漰���ķ�Ӧ�����мӳɷ�Ӧ����ȥ��Ӧ���ʴ�Ϊ���ӳɷ�Ӧ����ȥ��Ӧ��
��5�������Ϸ�����֪IΪCH3CH=CHCOOH���ʴ�Ϊ��CH3CH=CHCOOH��
��6��JΪCH3CH=CHCOOCH3������̼̼˫�����ɷ����Ӿ۷�Ӧ����Ӧ�ķ���ʽΪ
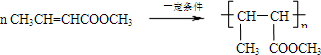
��
�ʴ�Ϊ��
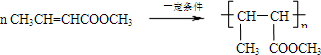
��
��7��EΪCH2=CHCH2CH2OH����E������ͬ�����ŵ�����ͬ���칹��Ϊ
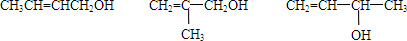
��
�ʴ�Ϊ��
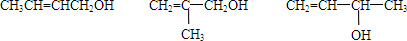
��

��ϰ��ϵ�д�
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
�����Ŀ









 +2��n-1��H2O
+2��n-1��H2O




