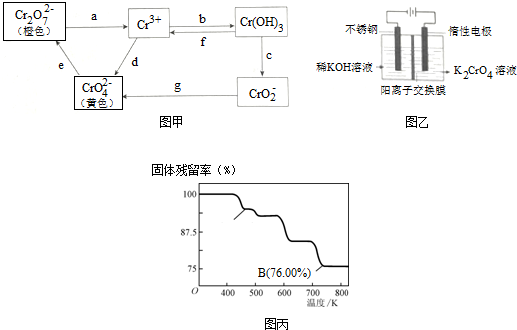
��Ŀ����
14������˵����ȷ���ǣ�������| A�� | ij�������ķ���ʽΪC10H14��������ʹ��ˮ��ɫ������ʹ����KMnO4��Һ��ɫ���ҷ��ӽṹ��ֻ��һ���������������������3�� | |
| B�� | ij�л���������ȫȼ�����ɵ����ʵ�����CO2��H2O������л���ķ���ʽһ��CnH2n | |
| C�� | ���顢��ȩ�����ᶼ������ͬ���칹�壬����ѣ�CH3OCH3��Ҳ��ͬ���칹�� | |
| D�� | HOCH2COOH�ȿɷ���ȡ����Ӧ��Ҳ�ɷ����Ӿ۷�Ӧ |
���� A�����жϸ÷��ӷ�����������ͨʽ���ٸ��������жϺ��еĹ����ţ����ж���ͬ���칹�壻
B�����л����п��ܺ���OԪ�أ�
C�������ѣ�CH3OCH3�����Ҵ���CH3CH2OH������ʽ��ͬ����ͬ���칹�壻
D�����д��ǻ����Ȼ����л�����������ش��жϣ�
��� �⣺A�������ķ���ʽC10H14����CnH��2n-6����ͨʽ��������ʹ��ˮ��ɫ������ʹKMnO4������Һ��ɫ�����Ժ��б�������������֪���÷��Ӻ��ж������������ֻ�ж϶��������ͬ���칹�弴�ɣ����������̼��ͬ���칹���У�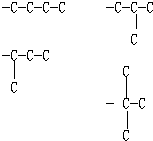 ����ͬϵ�����뱽��������Cԭ���ϱ��뺬��Hԭ�ӣ��ſɱ����Ը������������ʹ���Ը��������Һ��ɫ�����
����ͬϵ�����뱽��������Cԭ���ϱ��뺬��Hԭ�ӣ��ſɱ����Ը������������ʹ���Ը��������Һ��ɫ�����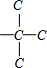 ���뱽��������Cԭ���ϲ���Hԭ�ӣ�����ʹ���Ը��������Һ��ɫ����˷���������������3�֣���A��ȷ��
���뱽��������Cԭ���ϲ���Hԭ�ӣ�����ʹ���Ը��������Һ��ɫ����˷���������������3�֣���A��ȷ��
B��ij�л���������ȫȼ�����ɵ����ʵ�����CO2��H2O��ֻ��˵���л�����C��H�ĸ�����Ϊ1��2����ȷ���Ƿ���OԪ�أ���B����
C�����顢��ȩ�����ᶼ������ͬ���칹�壬���Ƕ����ѣ�CH3OCH3�����Ҵ���CH3CH2OH������ʽ��ͬ����ͬ���칹�壬��C����
D��HOCH2COOH�к��д��ǻ����Ȼ������Է���ȡ����Ӧ��������Ӧ�����ܷ����Ӿ۷�Ӧ����D����ѡA��
���� �����漰�л������ʽ��ȷ����ͬ���칹�����д���л�������ʵ�֪ʶ�������ۺ�֪ʶ�Ŀ��飬�ѶȲ���

| A�� | ���������ϡ��ˮ�� H++NH3•H2O�TNH4++H2O | |
| B�� | �Է�̪Ϊָʾ���ñ�����ζ��մ���ҺCO32-+2H+�TCO2��+H2O | |
| C�� | �Ȼ�����Һ������������Һ��Ϻ���Ԫ����һ��ת��Ϊƫ������2Al3++7OH-�TAl��OH��3��+AlO2-+2H2O | |
| D�� | ��������ʯ�ҽ�����Ư�� Ca��OH��2+Cl2�TCa2++Cl-+ClO-+H2O |
| A�� | �����ᾧˮ�ⶨʵ���У�������ǯȡ��������������������ȴ | |
| B�� | ���Թܼмг��Թܼ���ʱ�����ֽ�����ס�Թܼеij����Ͷ̱� | |
| C�� | �ⶨ�кͷ�Ӧ�ķ�Ӧ��ʱ����NaOH��Һһ���Ե��������У����û��β��������������Һ | |
| D�� | ʵ���ҴӺ�������ȡ���ʵ�ķ����ǣ�ȡ�������ա��ܽ�����ˡ���ȡ������ |
| A�� | ʵ�����п�������ʯ����Ũ��ˮ��ϵķ�����ȡ�������� | |
| B�� | ���ζ�ʵ���У��ζ���װҺǰӦ�ô�װ��Һ��ϴ����ƿ�����ô�װҺ��ϴ | |
| C�� | �ڼ��ȵ�����£���ƿ�е�Һ�������Ҫ������ƿ�ݻ���3/4 | |
| D�� | ��KI��FeCl3��Һ��Ϻ���CCl4���ã��²�Һ�����ɫ��˵�������ԣ�Fe3+��I2 |
| A�� | ������ˮ�еμ�ŨH2SO4��KW���� | |
| B�� | ����AgCl��AgI���������Һ��c��Ag+����c��Cl-��=c��I-�� | |
| C�� | pH��ͬ�Ģ�CH3COONa��NaHCO3��NaClO������Һ��c��Na+�����٣��ڣ��� | |
| D�� | �����£�pH=2��������pH=12�İ�ˮ�������ϣ�������Һ�У�c��Cl-����c��NH4+����c��H+����c��OH-�� |
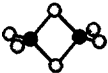 �������⻯��--���������ģ����ͼ��������ȡ���⻯﮵ķ�ӦΪ��2LiH+B2H6=2LiBH4
�������⻯��--���������ģ����ͼ��������ȡ���⻯﮵ķ�ӦΪ��2LiH+B2H6=2LiBH4